��Ŀ����
14�����л������٣�
 �ڣ�
�ڣ�
�ۣ�
 ��
�� ��
��
��1�����л�Ϊͬ���칹����Ǣ٢ڢۣ�������ţ�
��2��д���ܷ����ڴ������������¼��Ⱥ�������Ӧ�Ļ�ѧ����ʽ2CH3OH+O2$��_{��}^{����}$2HCHO+2H2O��
��3�����ڼ����������ܹ���HBr������Ӧ�����������飬����ۺ�HBr������Ӧ�����Ľṹ��ʽΪ
 ��
����4�����ֻ������1mol�ֱ�������Ʒ�Ӧ���������������С��ϵ�������������ͬ��
��5��������ϩ���Ҵ��Ļ������a L��ȫȼ������CO2��H2O�������������Ϊ3a L����ͬ״���£���������������ϩ���Ҵ��������ΪD��������ĸ��
A��1��1B��2��1 C��1��2 D������ȣ�
���� ��1�������л���Ľṹ��ʽ�жϷ���ʽ�ͽṹ���Դ��ж��Ƿ�ͬ���칹�壻
��2����ΪCH3OH��CH3OH���������ڴ������������·�Ӧ���ɼ�ȩ��ˮ��
��3����ΪCH3CH2OH��CH3CH2OH����HBr����ȡ����Ӧ���������飬�ǻ��ܱ���ԭ��ȡ����
��4�����ݹ�ϵʽ��2-COOH��H2��2-OH��H2�����
��5���������ʵ�����жϺ����������ʵ�����ͬ����CxHy�ĺ�����ȡ����x+$\frac{y}{4}$�����ʵ�����ͬ�ĺ�����������CxHyOz������ȡ����x+$\frac{y}{4}$-$\frac{z}{2}$���ݴ��ж����ʵ�����ͬ����ϩ���Ҵ��ĺ�������ͬ��
��� �⣺��1���٢ڢ۷���ʽ��ΪC8H8O2�����ṹ��ͬ����Ҫ�ǹ����Ų�ͬ������ͬ���칹�壻
�ʴ�Ϊ���٢ڢۣ�
��2����ΪCH3OH��CH3OH���������ڴ������������·�Ӧ���ɼ�ȩ��ˮ������ʽΪ��2CH3OH+O2 $��_{��}^{����}$ 2HCHO+2H2O��
�ʴ�Ϊ��2CH3OH+O2 $��_{��}^{����}$ 2HCHO+2H2O��
��3��CH3CH2OH����HBr����ȡ����Ӧ���������飬���ǻ��ܱ���ԭ��ȡ�������� ���ǻ��ܱ���ԭ��ȡ������
���ǻ��ܱ���ԭ��ȡ������ ��
��
�ʴ�Ϊ�� ��
��
��4�����ֻ����������1���ǻ���1���Ȼ�����2-COOH��H2��2-OH��H2������1mol�����ʷֱ�������Ʒ�Ӧ�����������������ͬ��
�ʴ�Ϊ�������������ͬ��
��5����ϩ���Ҵ��Ļ������a L��ȫȼ������CO2��H2O�������������Ϊ3a L����ͬ״���£�����1������������ȫȼ������3���������
��ϩ����ʽΪC2H4����x+$\frac{y}{4}$=2+1=3����1�����ϩ����3���������
�Ҵ��ķ���ʽΪC2H6O������x+$\frac{y}{4}$-$\frac{z}{2}$=2+$\frac{6}{4}$-$\frac{1}{2}$=3����1����Ҵ�����3���������
�ʻ����������ϩ���Ҵ��������������ȣ�
��ѡD��
���� ���⿼���л���Ľṹ�����ʣ���Ŀ�ѶȲ���ע������л�������ŵ����ʣ�����ͬ���칹����жϣ�

 �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| A�� | ��ϩ��2-��ϩҲ�ܷ������ƵĽṹ�仯 | |
| B�� | �ɽṹ�����ṹ�����������仯 | |
| C�� | �ṹ���ͽṹ����Ϊͬ���칹�� | |
| D�� | 1��2������ϩ������ԭ�Ӳ����ܴ���ͬһƽ���� |
| A�� | 10�� | B�� | 9�� | C�� | 8�� | D�� | 7�� |
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |

| A�� | ���л�����Է����������ӳɡ��Ӿۡ�ȡ���ȷ�Ӧ | |
| B�� | ���л�������NaOH��Һ��Ӧ����1mol���л���������2molNaOH | |
| C�� | ���л���ķ���ʽΪC12H12O5������C11H10O5 һ����Ϊͬϵ�� | |
| D�� | ���л������������̼ԭ�Ӳ����ܶ���ͬһƽ���� |
�������ˮ�м������BaCl2��Һ�����ˣ�
����������Һ�м������Na2CO3��Һ�����ˣ�
����Һ���������pH�����һ�ξ�����ˮ��
��֪�����̢����ɵIJ��ֳ��������ܽ�ȣ�20��/g�������
���������գ�
| CaSO4 | Mg2��OH��2CO3 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 2.5��10-4 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
��2�����Fe3+�Ƿ�����ķ�����ȡ��������II�����Һ���Թ��У��μӼ���KSCN��Һ������Һ����죬˵��Fe3+�ѳ�������֮û������
��3�����̢�ѡ��BaCl2����ѡ��CaCl2�������ñ������ݽ���ԭ��BaSO4���ܽ�ȱ�CaSO4��С���ɽ�SO42-�������꣮
��4����ȥMg2+�����ӷ���ʽ��2Mg2++2CO32-+H2O�TMg2��OH��2CO3��+CO2����
��5�����Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�ԭ������BaCO3��CaCO3��Mg2��OH��2CO3�У�BaCO3���ܽ�������Ba2+������ȫ����˵��Mg2+��Ca2+Ҳ������ȫ��
�ڶ��ξ���Ҫ��ȥ����I-��IO3-��NH4+��Ca2+��Mg2+������ʾ�����£�

��6�����̢���ȥ��������NH4+��I-��
��7������VI�У��ڵ��۵�����������NaOH����ϻ�ѧƽ��ԭ������H+�������ϵõ��ӱ��H2�ݳ���ʹH2O?H++OH-����ƽ�������ƶ���OH-��������Ũ������Na+���������ƶ������NaOH�����������ɣ�
| A�� | �����۵�ĸߵͣ�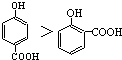 | |
| B�� | Ӳ���ɴ�С�����ʯ��̼���裾����� | |
| C�� | �۵��ɸߵ��ͣ�Na��Mg��Al | |
| D�� | �������ɴ�С��NaF��NaCl��NaBr��NaI |
 �������ƣ�NaNO2����һ����Ҫ�Ĺ�ҵ�Σ��׳��⣬������ˮ�������Ҵ�����¶�ڿ����л���������Ӧ���������ƣ�
�������ƣ�NaNO2����һ����Ҫ�Ĺ�ҵ�Σ��׳��⣬������ˮ�������Ҵ�����¶�ڿ����л���������Ӧ���������ƣ�
