��Ŀ����
6���������¼����л����CH4��CH3CH2OH��
 �ܹ����CH3COOH ��
�ܹ����CH3COOH �� ��
�� ��
�� �����
������������������������ʰ�Ҫ��ش��������⣺
��1����Է�������Ϊ44�������Ľṹ��ʽΪCH3CH2CH3��
��2�������к���14����ԭ�ӵ������ķ���ʽ��C6H14��
��3����ۻ�Ϊͬ���칹����Ǣߣ�����ţ���
��4������������ζ��������ȡ�����л�����������������������Һ�巢��һȡ����Ӧ�Ļ�ѧ����ʽ
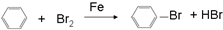 ��
����5���á�������ʾ�٢ۢܢ��۷е�ߵ�˳�ܣ��ۣ���٣�����ţ���
��6���л�����ڼ��������º�CuO��Ӧ�Ļ�ѧ����ʽ
 ��
����7����120�棬1.01��105Pa�����£�ij����̬����������O2��ȫ��Ӧ��÷�Ӧǰ����������û�з����ı䣬������Ǣ٣�����ţ�������Ϊͬϵ���ϵ��
��8���л���ݺ͢���һ�������·�����Ӧ�Ļ�ѧ����ʽ��
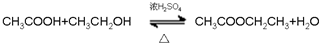 ��
��
���� ��1����2������������ͨʽCnH2n+2�����㣻
��3������ʽ��ͬ���ṹ��ͬ���л��ﻥΪͬ���칹�壻
��4������������ζ��������ȡ�������л�����Һ�巢��ȡ����Ӧ����Ϊ���������嵥�ʷ�Ӧ�����屽��
��5��̼ԭ�Ӹ���Խ�࣬�е�Խ����̼ͬԭ�Ӹ�����������֧����ķе�ͣ�
��6���Ҵ���CuO��Ӧ������ȩ��Cu��ˮ��
��7����������ˮΪ���壬��Ӧǰ����������û�з����ı䣬��ǰ������Ļ�ѧ��������ȣ������������������г��ȼ�յ�ͨʽ���㣻
��8���������Ҵ�����������Ӧ��������������ˮ��
��� �⣺��1��������ͨʽΪ��CnH2n+2����Է�������Ϊ44����������12n+2n+2=44������n=3���������ķ���ʽΪC3H8���ṹ��ʽΪCH3CH2CH3��
�ʴ�Ϊ��CH3CH2CH3��
��2����������ͨʽCnH2n+2����2n+2=14�����n=6������ΪC6H14��
�ʴ�Ϊ��C6H14��
��3����ۻ�Ϊͬ���칹����Ǣߣ����߷���ʽ��ͬ���ṹ��ͬ��
�ʴ�Ϊ���ߣ�
��4������������ζ��������ȡ�����л���Ϊ������������������������Һ�巢��һȡ����Ӧ�Ļ�ѧ����ʽΪ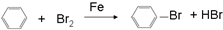 ��
��
�ʴ�Ϊ��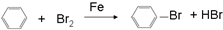 ��
��
��5��̼ԭ�Ӹ���Խ�࣬�е�Խ����̼ͬԭ�Ӹ�����������֧����ķе�ͣ���е�Ϊ�ܣ��ۣ���٣�
�ʴ�Ϊ���ܣ��ۣ���٣�
��6���Ҵ���CuO��Ӧ������ȩ��Cu��ˮ����ӦΪ ��
��
�ʴ�Ϊ�� ��
��
��7����120�棬1.01��105Pa�����£����ɵ�ˮΪ��̬����CxHy+��x+$\frac{y}{4}$��O2$\stackrel{��ȼ}{��}$xCO2+$\frac{y}{2}$H2O��g������ 1+��x+$\frac{y}{4}$��=x+$\frac{y}{2}$�����y=4��������ʽ����ԭ����ĿΪ4��
Ϊ���飬����Ϊͬϵ�
�ʴ�Ϊ���٣�ͬϵ�
��8���������Ҵ�����������Ӧ��������������ˮ���÷�ӦΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л���Ľṹ�����ʣ����չ����������ʵĹ�ϵ���л���ӦΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

| A�� | ��������һ��Ԫ����ɵ����ʣ�����һ��Ԫ����ɵ�����һ���ǵ��� | |
| B�� | ��Ԫ������Ԫ��ԭ�ӵ�������������ͬ�����Զ��߾������ƵĻ�ѧ���� | |
| C�� | ��Һ���о���������������������С���������ʵ���������һ����С | |
| D�� | ����Һ��ʹ��̪��Һ��죬�����̪��Һ�����һ���Ǽ���Һ |
| A�� | C��N��O | B�� | S��P��Si | C�� | F��O��Br | D�� | H��Ca��Al |
| H2O2��Ũ�ȣ����������� | �¶ȣ��棩 | ���� | |
| A | 5 | 10 | ��ʹ�� |
| B | 5 | 15 | ʹ��FeCl3 |
| C | 15 | 20 | ��ʹ�� |
| D | 15 | 30 | ʹ��MnO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ױ������е�����ԭ�ӿ��ܹ�ƽ�� | |
| B�� | CH2�TCH-C6H5�����е�����ԭ�ӿ��ܹ�ƽ�� | |
| C�� | ��������е�����ԭ�ӿ��ܹ�ƽ�� | |
| D�� | ���ȼ������Ϊ��������ṹ |
| �� | E | A | B | ||
| C | �� | D |
��2��A��B�����������Ϊ7��16����ԭ�ӷ��ӣ��÷����ͷ��ڿ����п�������Ļ�������
�У����꣨��⻯ѧ����������дһ�֣���
��3��A��C��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��Na3N+4H2O=3NaOH+NH3•H2O��
��4��ͬ��ͬѹ�£���a L A�⻯��������b L D���⻯������ͨ��ˮ�У���������Һ��pH=7����a��b�������������=����
��5��д��F�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��6����֪һ������E��������B2 ��g����ȼ�գ�����ܵIJ��P������ϵ��ͼ1��ʾ����д��һ��������EB2��g�� ��E��s����Ӧ����EB��g�����Ȼ�ѧ����ʽCO2��g��+C��s��=2CO��g����H=+172.5kJ/mol

��7������D��G��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ�����ݲ���������ķ�Ӧԭ��������Ƶ�ԭ�����ͼ2��ʾ���䷴Ӧ��������ӦʽΪFe3++e-=Fe2+��
 ��
�� ���������İ���ͭ���ӣ�[Cu��NH3��4]2+�е�����NH3������Cl-ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ����[Cu��NH3��4]2+�Ŀռ乹��Ϊƽ�������Σ�
���������İ���ͭ���ӣ�[Cu��NH3��4]2+�е�����NH3������Cl-ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ����[Cu��NH3��4]2+�Ŀռ乹��Ϊƽ�������Σ�
 ����Ԫ�����������ǿ��Ԫ���γɵĻ�����ĵ���ʽΪ
����Ԫ�����������ǿ��Ԫ���γɵĻ�����ĵ���ʽΪ ���û������д��ڵĻ�ѧ��Ϊ���Ӽ� ��ѡ����Ӽ������ۼ�������
���û������д��ڵĻ�ѧ��Ϊ���Ӽ� ��ѡ����Ӽ������ۼ�������