ƒøƒ⁄»ð
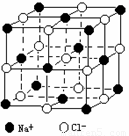
æßÃÂæþÃÂπÊ‘Úµƒº∏∫ŒÕ‚–Œ£¨æßÃÂ÷–◊Óª˘±æµƒ÷ÿ∏¥µ•Œª≥∆Œ™æß∞˚£ÆNaClæßÃÂΩ·ππ»ÁÕºÀ˘ æ£Æ“—÷™FexOæßÃÂæß∞˚Ω·ππŒ™NaCl–Õ£¨”…”⁄æßû±œð£¨x÷µ–°”⁄1£Æ≤‚÷™FexOæßÃÂ√Ð∂»Œ™¶—=5.71g°§cm£≠3£¨æß∞˚±þ≥§Œ™4.28°¡10£≠10m£Æ(1) FexO÷–x÷µ(æ´»∑÷¡0.01)Œ™________£Æ
(2)æßÃÂ÷–µƒFe∑÷±Œ™Fe2+°¢Fe3+£¨‘⁄Fe2+∫ÕFe3+µƒ◊Ð ˝÷–£¨Fe2+À˘’º∑÷ ˝£®”√–° ˝±Ì 棨活∑÷¡0.01£©Œ™________£Æ
(3)¥ÀæßÃÂªØ—ß ΩŒ™________________£Æ
(4)”΃≥∏ˆFe2+(ªÚFe3+)æý¿Î◊ÓΩ¸«“µ»æý¿ÎµƒO2£≠Œß≥…µƒø’º‰º∏∫Œ–Œ◊¥ «________£Æ
(5)‘⁄æßÃÂ÷–£¨Ã˙‘™Àÿµƒ¿Î◊”º‰◊Ó∂Ãæý¿ÎŒ™________m£Æ

¥∞∏£∫
Ω‚Œˆ£∫
÷ æ£∫
Ω‚Œˆ£∫
| (1)”…NaClæßÃÂΩ·ππø…÷™£¨1∏ˆNaClæß∞˚”…8∏ˆ–°¡¢∑ΩÃÂππ≥…£¨√ø∏ˆ–°¡¢∑Ωõƒ8∏ˆ∂•µ„∑÷±”…4∏ˆNa+°¢4∏ˆCl£≠œý¡⁄’ºæð£Æ√ø∏ˆNa+°¢Cl£≠∂º÷ª”– (3)”…”⁄Fe2+Œ™0.76£¨‘ÚFe3+Œ™(0.92£≠0.76)=0.16£¨π ªØ—ß ΩŒ™£∫ (4)”…NaClæßÃÂΩ·ππø…Õ∆÷™£¨”ÎFe2+(ªÚFe3+)æý¿Î◊ÓΩ¸«“µ»æý¿ÎµƒO2£≠”–6∏ˆ£¨’‚6∏ˆO2£≠À˘Œß≥…µƒº∏∫Œ–Œ◊¥Œ™’˝∞À√Êà(5)…ËFexO÷–Fe°™Fe◊Ó∂õƒæý¿ÎŒ™r£Æ”…FexOæßõƒ“ª∏ˆ∆Ω√ÊÕº(”“ÕºÀ˘ æ)ø…÷™£¨‘⁄÷±Ω«»˝Ω«–ŒABC÷–£¨–±±þBCŒ™r£¨∂¯AB=AC=æß∞˚±þ≥§/2£Æ“Ú¥Àr2=AB2+AC2£¨r=
|
÷ æ£∫
| ¿ý±»NaClµƒæßÃÂΩ·ππ£¨ø…“‘À„≥ˆFexOµƒƒ¶∂˚÷ ¡ø£¨¥”∂¯«Ûµ√xµƒæþÃÂ÷µ£Æ
|

¡∑œ∞≤·œµ¡–¥∞∏
œýπÿƒø



 (4)æßÃÂ÷–,Fe¿Î◊”º‰◊ÓΩ¸æý¿ÎŒ™ cm
(4)æßÃÂ÷–,Fe¿Î◊”º‰◊ÓΩ¸æý¿ÎŒ™ cm