��Ŀ����
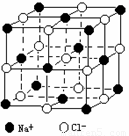
����������ļ������Σ���������������ظ���λ��Ϊ������NaCl����ṹ��ͼ��ʾ����֪FexO���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵС��1����֪FexO�����ܶ�Ϊ��=5.71g��cm��3�������߳�Ϊ4.28��10��10m��(1) FexO��xֵ(��ȷ��0.01)Ϊ________��
(2)�����е�Fe�ֱ�ΪFe2+��Fe3+����Fe2+��Fe3+�������У�Fe2+��ռ����(��С����ʾ����ȷ��0.01)Ϊ________��
(3)�˾��廯ѧʽΪ________________��
(4)��ij��Fe2+(��Fe3+)��������ҵȾ����O2��Χ�ɵĿռ伸����״��________��
(5)�ھ����У���Ԫ�ص����Ӽ���̾���Ϊ________m��

�𰸣�
������
��ʾ��
������
| (1)��NaCl����ṹ��֪��1��NaCl������8��С�����幹�ɣ�ÿ��С�������8������ֱ���4��Na+��4��Cl������ռ�ݣ�ÿ��Na+��Cl����ֻ��1��8ռ����С�������У����ÿ��С�����庬Na+��(1��8)��4=1��2������Cl��Ϊ(1��8)��4=1��2��������NaCl������8��С��������ɣ���ÿ��������Na��Cl����8��(1��2)=4����ͬ������Fex0��������4��Fex0������1mol�����к���4mol FexO����FexO��Ħ������ΪM�����У�4M=��VNA��M=��VNA��4=[5.71��(4.28��10��8)��3��6.02��1023]��4=67.4(g��mol��1)����Fex0�У�55.9x+16=67.4����x=0.92 (2)�躬y��Fe2+������(0.92��y)��Fe3+�������������ϼ۴�����Ϊ�㣬�ã�2y+3(0.92��y)=2�����y=0.76(��)����Fe3+��ռ����Ϊ��0.76��0.92=0.826 (3)����Fe2+Ϊ0.76����Fe3+Ϊ(0.92��0.76)=0.16���ʻ�ѧʽΪ�� (5)��FexO��Fe��Fe��̵ľ���Ϊr����FexO�����һ��ƽ��ͼ(��ͼ��ʾ)��֪����ֱ��������ABC�У�б��BCΪr����AB=AC=�����߳�/2�����r2=AB2+AC2��r=
|
��ʾ��
| ���NaCl�ľ���ṹ���������FexO��Ħ���������Ӷ����x�ľ���ֵ��
|

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



 (4)������,Fe���Ӽ��������Ϊ cm
(4)������,Fe���Ӽ��������Ϊ cm