��Ŀ����
3��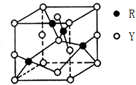 X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��Yͬ���ڣ�X��̬ԭ�ӵ������������Ǵ�����2����Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ�R+���ӵ�3d���ȫ������
X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��Yͬ���ڣ�X��̬ԭ�ӵ������������Ǵ�����2����Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ�R+���ӵ�3d���ȫ��������ش��������⣺
��1��Z3+���ӵĺ�������Ų�ʽ��1s22s22p6��
��2����ѧ�ҳɹ����ڸ�ѹ�½�XY2ת��Ϊ��������SiO2�ṹ��ԭ�Ӿ��壬�þ�����Xԭ�ӵ��ӻ����������sp3��X��Z��R�ĵ��ʷֱ�������Y2��ַ�Ӧ���ò�����۵��ɸߵ��͵�˳����Al2O3��CuO��CO2�����ѧʽ��
��3����Y��R�γɵ�ij������ľ����ṹ����ͼ��ʾ���仯ѧʽ��CuO��
��4�������£�pH��ͬ��NaZY2��Na2XY3������Һ�����ʵ���Ũ�Ƚϴ����Na2CO3�����ѧʽ��
��5�����������Ƶ�ˮ��Һ��μ���RCl2��ˮ��Һ�У��ټ�������Ũ������ȣ��õ����ܵİ�ɫ����RCl���÷�Ӧ�����ӷ���ʽ��SO32-+2Cu2++2Cl-+H2O=2CuCl��+SO42-+2H+��
���� X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��̬ԭ�ӵ������������Ǵ�����2�����������ຬ��8�����ӣ���X�����������Ӳ㣬�����Ϊ4�����ӣ�����XΪCԪ�أ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ���ZΪAlԪ�أ�X��Yͬ���ڣ���YΪ�ڶ����ڣ�Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p4��ΪOԪ�أ�R+���ӵ�3d���ȫ�������ĺ�������Ų�ʽΪ��1s22s22p63s23p63d10��Rԭ�ӵ�ԭ������Ϊ29����RΪCuԪ�أ��ݴ˽��н��
��� �⣺X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��̬ԭ�ӵ������������Ǵ�����2�����������ຬ��8�����ӣ���X�����������Ӳ㣬�����Ϊ4�����ӣ�����XΪCԪ�أ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ���ZΪAlԪ�أ�X��Yͬ���ڣ���YΪ�ڶ����ڣ�Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p4��ΪOԪ�أ�R+���ӵ�3d���ȫ�������ĺ�������Ų�ʽΪ��1s22s22p63s23p63d10��Rԭ�ӵ�ԭ������Ϊ29����RΪCuԪ�أ�
��1��ZΪAlԪ�أ������Ӻ����������Ϊ10�������ӵĺ�������Ų�ʽΪ��1s22s22p6��
�ʴ�Ϊ��1s22s22p6��
��2��XY2ΪCO2��CO2�ڸ��¸�ѹ�����γɵľ����������SiO2�ṹ��ԭ�Ӿ��壬�þ�����Cԭ���γ�4��C-O��������Cԭ�Ӻ���4�۲���Ӷԣ�����Cԭ�ӹ�����ӻ�����Ϊsp3��
X��Z��R�ֱ�ΪC��Al��CuԪ�أ�Y2Ϊ��������X��Z��R�ĵ��ʷֱ�������Y2��ַ�Ӧ���ò���ֱ�Ϊ��������̼��������������ͭ��������̼�γɵľ���Ϊ���Ӿ��壬��е���ͣ�����ͭ���������������Ӿ��壬���������������Ӽ��϶̣�����е�ϸߣ��������ߵķе�ߵ�Ϊ��Al2O3��CuO��CO2��
�ʴ�Ϊ��sp3��Al2O3��CuO��CO2��
��3��YΪOԪ�ء�RΪCuԪ�أ���O��Cu�γɵ�ij������ľ����ṹ����ͼ��ʾ��Y����Ŀ=8��$\frac{1}{8}$+4��$\frac{1}{4}$+2��$\frac{1}{2}$+1=4��Rԭ����ĿΪ4������Y��R��������Ŀ֮��Ϊ4��4=1��1�����Ըû�����Ļ�ѧʽΪCuO��
�ʴ�Ϊ��CuO��
��4��NaZY2��Na2XY3�ֱ�ΪNaAlO2��Na2CO3��̼��������ӵ����Դ���������������pH��ͬʱ��̼������Һ��Ũ�ȴ���ƫ�����ƣ��ʴ�Ϊ��Na2CO3��
��5����ɫ����ΪCuCl�����߷�����������ԭ��Ӧ���÷�Ӧ�����ӷ���ʽΪSO32-+2Cu2++2Cl-+H2O=2CuCl��+SO42-+2H+��
�ʴ�Ϊ��SO32-+2Cu2++2Cl-+H2O=2CuCl��+SO42-+2H+��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ�ƶϸ�Ԫ������Ϊ���ؼ���ע������ԭ�ӽṹ��Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ�����Ӧ�û���֪ʶ��������

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�| A�� | ����ٵĻ�ѧ����ʽΪ��SiO2+C$\frac{\underline{\;����\;}}{\;}$Si+CO2�� | |
| B�� | ����٢ڢ���ÿ���ɻ�Ӧ1mol Si��ת��4mol���� | |
| C�� | ����������������ᷴӦ�����費��������ᷴӦ | |
| D�� | SiHCl3���е�33.0�棩�к���������SiCl4���е�67.6�棩��ͨ����������ᴿSiHCl3 |
| A�� | NC13��N-C1��������CCl4��C-C1�������� | |
| B�� | NC13�����е�����ԭ�Ӿ��ﵽ8�����ȶ��ṹ | |
| C�� | NCl3�����Ǽ��Է��� | |
| D�� | NBr3�ķе��NCl3�ķе�� |
| A�� | �ñ����������Һ���������� | |
| B�� | ����ʯ�Ҹ��ﰱ�� | |
| C�� | �����Ƶ�����ʢװŨ���� | |
| D�� | �ñ���̼������Һ�ռ�ʵ������ȡ���������� |
| A�� | W��X��Y�γɵļ����ӣ���뾶��С��ϵΪW��X��Y | |
| B�� | M��WԪ���γɵļ��⻯����ȶ��ԣ�W��M | |
| C�� | X��ͬ�����н�������ǿ��Ԫ�� | |
| D�� | Z������������Ӧ��ˮ�����Ũ��Һ����Y�ĵ����ڳ����¾��ҷ�Ӧ |

��1���ϳɼ״��ķ�Ӧ���������������仯��ͼ1��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽCO��g��+2H2��g��?CH3OH��g����H=-��b-a��kJ/mol��
��2��ʵ������lL�ܱ������н���ģ��ϳ�ʵ�飮��1molCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£����������ݵ�λ��mol•L-l��
| �¶�\ʱ�� | 10min | 20min | 30min | 40min | 50min | 60min |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
��500��ʱƽ�ⳣ��K����ֵΪ25��
��300��ʱ�����������ݻ�ѹ����ԭ����$\frac{1}{2}$���������������������£���ƽ����ϵ������Ӱ����cd��ѡ���ţ���
a��c��H2����С
b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH�����ʵ�������
d������ƽ��ʱ$\frac{c��{H}_{2}��}{c��C{H}_{3}OH��}$��С
��3����ͼ2�Ǽ״�ȼ�ϵ�ع�����ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
�ټ��и����ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
������A�������������ڱ�״���µ����Ϊ2.24l��
�۷�Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ300mL5.0mol•L-lNaOH ��Һ��
 �ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������l0%��NaOH��Һ���õ�������������¼���£�
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������l0%��NaOH��Һ���õ�������������¼���£�| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ�