��Ŀ����
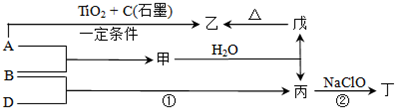
ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�A��B��H�����壬����֮��������ת����ϵ������Ӧ�����ɵ�ˮ����ȥ��

��ش��������⣺
��1������F�Ļ�ѧʽ��
��2��C�������ճ������п���
��3��д����Ӧ�ٵĻ�ѧ����ʽ��
��4��д����Ӧ�ڵ����ӷ���ʽ��

��ش��������⣺
��1������F�Ļ�ѧʽ��
HCl
HCl
����2��C�������ճ������п���
��������Ư�ף�
��������Ư�ף�
������3��д����Ӧ�ٵĻ�ѧ����ʽ��
2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O
2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O
����4��д����Ӧ�ڵ����ӷ���ʽ��
CO2+Ca2++2OH-�TCaCO3��+H2O
CO2+Ca2++2OH-�TCaCO3��+H2O
��������B�������ʯ��ˮ��Ӧ���ɳ�����ӦΪCO2����EΪCaCO3����������ʯ��ˮ��Ӧ�����廹����������AӦΪCl2���ɴ˿�֪CΪCa��ClO��2��FΪHCl��DΪCaCl2��GΪHClO��HΪO2����϶�Ӧ���ʵ������Լ���ĿҪ��ɽ����⣮
����⣺B�������ʯ��ˮ��Ӧ���ɳ�����ӦΪCO2����EΪCaCO3����������ʯ��ˮ��Ӧ�����廹����������AӦΪCl2���ɴ˿�֪CΪCa��ClO��2��FΪHCl��DΪCaCl2��GΪHClO��HΪO2��
��1�������Ϸ�����֪FΪHCl���ʴ�Ϊ��HCl��
��2��CΪCa��ClO��2��������HClO������ǿ�����Ժ�Ư���ԣ�������������Ư�ף��ʴ�Ϊ����������Ư�ף���
��3����Ӧ�ٵĻ�ѧ����ʽΪ2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O��
��4����Ӧ�ڵ����ӷ���ʽΪCO2+Ca2++2OH-�TCaCO3��+H2O���ʴ�Ϊ��CO2+Ca2++2OH-�TCaCO3��+H2O��
��1�������Ϸ�����֪FΪHCl���ʴ�Ϊ��HCl��
��2��CΪCa��ClO��2��������HClO������ǿ�����Ժ�Ư���ԣ�������������Ư�ף��ʴ�Ϊ����������Ư�ף���
��3����Ӧ�ٵĻ�ѧ����ʽΪ2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O��
��4����Ӧ�ڵ����ӷ���ʽΪCO2+Ca2++2OH-�TCaCO3��+H2O���ʴ�Ϊ��CO2+Ca2++2OH-�TCaCO3��+H2O��
���������⿼��������ƶϣ���Ŀ�ۺϿ���Ԫ�ػ�����������Լ�Ӧ�ã�������ѧ�������������ۺ����û�ѧ֪ʶ�Ŀ��飬ͻ�ƿ�ΪB��A�Լ�G�����ת����ϵ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
| |||||||||||||||||||
 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����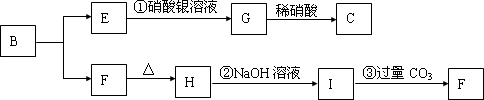
 ��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��
��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��