��Ŀ����
����Ҫ240mL1.0mol/L��NaCl��Һ����ش��������⣺
��1��Ӧѡ�õ�����ƿ���Ϊ�� mL��Ӧ����NaCl g��
������ƿ���ձ�������������Ͳ�����ÿɲ��ã����Լ�ƿ���Ҫ�����������У� �� ��
��2������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸ��һ�Σ� ��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ�У���
B��ȷ���������NaCl���������ձ��У���������ˮ��Լ30mL�����ò������������裬ʹ�����ܽ�
C������ȴ���NaCl��Һע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ������͵�ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
��3������A�У���ϴ��Һ��ע������ƿ�У���Ŀ���� ��
��4�����ƹ����м�����ˮ���������˿̶ȣ�����ҺŨ�Ƚ� ���㽫��δ����� ��
��1��Ӧѡ�õ�����ƿ���Ϊ��
������ƿ���ձ�������������Ͳ�����ÿɲ��ã����Լ�ƿ���Ҫ�����������У�
��2������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸ��һ�Σ�
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ�У���
B��ȷ���������NaCl���������ձ��У���������ˮ��Լ30mL�����ò������������裬ʹ�����ܽ�
C������ȴ���NaCl��Һע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ������͵�ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
��3������A�У���ϴ��Һ��ע������ƿ�У���Ŀ����
��4�����ƹ����м�����ˮ���������˿̶ȣ�����ҺŨ�Ƚ�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺ʵ����
��������1����������ƿֻ��һ���̶��ߣ�ֻ�������������Ӧ���������Һ������n=CV��m=nM�����㣻�������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��3���������ϴ��Һ��ע������ƿ�����������ʵ���ʧ��������
��4����ˮ�����̶��ߣ�����Һ�����ƫ��Ũ��ƫС���������ֲ����ݴ˷�����
��2���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��3���������ϴ��Һ��ע������ƿ�����������ʵ���ʧ��������
��4����ˮ�����̶��ߣ�����Һ�����ƫ��Ũ��ƫС���������ֲ����ݴ˷�����
���
�⣺��1����������ƿֻ��һ���̶��ߣ�ֻ�������������Ӧ���������Һ�����õ�����ƿ�Ĺ����100ml��250ml��500ml��1000ml����Ӧѡ��250ml������ƿ��
����ѡ��250ml������ƿ�������Ƶ���250ml��Һ������Ҫ��NaCl�����ʵ���n=cV=0.25L��1.0mol/L=0.25mol��m=nM=0.25mol��58.5g/mol=14.6g��
���������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������������ƽ����ͷ�ιܣ�
�ʴ�Ϊ��250��14.6��������ƽ����ͷ�ιܣ�
��2�����Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ȷ�IJ���˳����BCAFED���ʴ�Ϊ��BCAFED��
��3�����ϴ��Һ��ע������ƿ�����������ʵ���ʧ���������ҺŨ��ƫ�ͣ��ʴ�Ϊ������������ʧ��
��4����ˮ�����̶��ߣ�����Һ�����ƫ������ҺŨ�Ȼ�ƫ�ͣ����ڴ˲���ʧ�����ֲ�����Ӧ�������ƣ��ʴ�Ϊ��ƫ�ͣ��������ƣ�
����ѡ��250ml������ƿ�������Ƶ���250ml��Һ������Ҫ��NaCl�����ʵ���n=cV=0.25L��1.0mol/L=0.25mol��m=nM=0.25mol��58.5g/mol=14.6g��
���������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������������ƽ����ͷ�ιܣ�
�ʴ�Ϊ��250��14.6��������ƽ����ͷ�ιܣ�
��2�����Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ȷ�IJ���˳����BCAFED���ʴ�Ϊ��BCAFED��
��3�����ϴ��Һ��ע������ƿ�����������ʵ���ʧ���������ҺŨ��ƫ�ͣ��ʴ�Ϊ������������ʧ��
��4����ˮ�����̶��ߣ�����Һ�����ƫ������ҺŨ�Ȼ�ƫ�ͣ����ڴ˲���ʧ�����ֲ�����Ӧ�������ƣ��ʴ�Ϊ��ƫ�ͣ��������ƣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��25�棬ijϡ��Һ����ˮ���������c��H+�� Ϊ10-13mol/L�������й���Һ��������ȷ���ǣ�������
| A������Һһ�������� |
| B������Һһ���ʼ��� |
| C������Һ��pH����ԼΪ1 |
| D������Һ��pH����ԼΪ13��Ҳ����Ϊ1 |
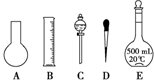 ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ500mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ500mL��������������Һ����������ش��������⣺
