��Ŀ����
ԭ�Ӻ˴Ź����ף�PMR�����о��л�������ṹ����Ҫ����֮һ�������о��Ļ���������У�����λ����ȫ��ͬ����ԭ�ӣ�����Hԭ�ӣ��ں˴Ź������г���ͬһ���źŷ壬���з��ǿ�������Hԭ�ӵ���Ŀ�����ȣ��� ��ȩ��CH3CHO���ں˴Ź���������2���źŷ壬��ǿ��֮��Ϊ3��1��
��1��CH3CH2COOH��PMR���й۲쵽���ַ��ǿ��֮��Ϊ
��2��ʵ���п��Ը���PMR���й۲쵽����ԭ�Ӹ����ķ�ֵ�����ȷ���л���Ľṹ����֪�л���W��C3H6O3������NaHCO3��Ӧ���ҷ�ֵǿ��֮��Ϊ3��1��1��1�����ƶ��л���W�к��еĹ�������
��3����֪�л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ����
�ٷ�����������Է���������С���л���A��
����A�����ᣨ ������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪ
������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪ
��1��CH3CH2COOH��PMR���й۲쵽���ַ��ǿ��֮��Ϊ
3��2��1
3��2��1
����2��ʵ���п��Ը���PMR���й۲쵽����ԭ�Ӹ����ķ�ֵ�����ȷ���л���Ľṹ����֪�л���W��C3H6O3������NaHCO3��Ӧ���ҷ�ֵǿ��֮��Ϊ3��1��1��1�����ƶ��л���W�к��еĹ�������
�ǻ����Ȼ�
�ǻ����Ȼ�
�������ƣ������Ӧ�Ľṹ��ʽΪCH3CH��OH��COOH
CH3CH��OH��COOH
����3����֪�л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ����
�ٷ�����������Է���������С���л���A��
HCHO
HCHO
����д�ṹ��ʽ��������A�����ᣨ
 ������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪ
������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪHOCH2CH��OH��CHO
HOCH2CH��OH��CHO
��CO��CH2OH��2
CO��CH2OH��2
����������1���˴Ź����������źŷ�ǿ��֮�ȵ��ڻ�ѧ������ͬHԭ����Ŀ֮�ȣ�
��2���л���W��C3H6O3������NaHCO3��Ӧ������-COOH����ֵǿ��֮��Ϊ3��1��1��1������4��Hԭ�ӣ���Hԭ����Ŀ�ֱ�Ϊ3��1��1��1����Ӧ����-OH�������ݴ���д��
��3���л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ������HԪ�ص�����������ͬ��C3H6O3�����ʽΪHCHO��
�����ʽΪ��Է���������С���л���A�Ľṹ��ʽ��
����A�����ᣨ ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��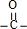 ���ݴ���д���
���ݴ���д���
��2���л���W��C3H6O3������NaHCO3��Ӧ������-COOH����ֵǿ��֮��Ϊ3��1��1��1������4��Hԭ�ӣ���Hԭ����Ŀ�ֱ�Ϊ3��1��1��1����Ӧ����-OH�������ݴ���д��
��3���л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ������HԪ�ص�����������ͬ��C3H6O3�����ʽΪHCHO��
�����ʽΪ��Է���������С���л���A�Ľṹ��ʽ��
����A�����ᣨ
 ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��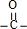 ���ݴ���д���
���ݴ���д�������⣺��1��CH3CH2COOH��3�ֻ�ѧ������ͬ��Hԭ�ӣ�3��Hԭ����Ŀ�ֱ�Ϊ3��2��1������PMR���й۲쵽���ַ��ǿ��֮��Ϊ3��2��1��
�ʴ�Ϊ��3��2��1��
��2���л���W��C3H6O3������NaHCO3��Ӧ������-COOH����ֵǿ��֮��Ϊ3��1��1��1������4��Hԭ�ӣ���Hԭ����Ŀ�ֱ�Ϊ3��1��1��1����Ӧ����-OH��������ṹΪCH3CH��OH��COOH��
�ʴ�Ϊ���ǻ����Ȼ���CH3CH��OH��COOH��
��3���л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ������HԪ�ص�����������ͬ��C3H6O3�����ʽΪHCHO��
����Է���������С���л���A�Ľṹ��ʽΪHCHO���ʴ�Ϊ��HCHO��
����A�����ᣨ ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��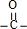 ������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2��
������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2��
�ʴ�Ϊ��HOCH2CH��OH��CHO��CO��CH2OH��2��
�ʴ�Ϊ��3��2��1��
��2���л���W��C3H6O3������NaHCO3��Ӧ������-COOH����ֵǿ��֮��Ϊ3��1��1��1������4��Hԭ�ӣ���Hԭ����Ŀ�ֱ�Ϊ3��1��1��1����Ӧ����-OH��������ṹΪCH3CH��OH��COOH��
�ʴ�Ϊ���ǻ����Ȼ���CH3CH��OH��COOH��
��3���л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ������HԪ�ص�����������ͬ��C3H6O3�����ʽΪHCHO��
����Է���������С���л���A�Ľṹ��ʽΪHCHO���ʴ�Ϊ��HCHO��
����A�����ᣨ
 ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��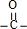 ������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2��
������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2���ʴ�Ϊ��HOCH2CH��OH��CHO��CO��CH2OH��2��
���������⿼���л���ṹ��ȷ���������ŵ����ʡ��˴Ź������ס�ͬ���칹����д�ȣ��Ѷ��еȣ����չ����ŵ������ǹؼ���

��ϰ��ϵ�д�
�����Ŀ