��Ŀ����
������Ksp��AgCl��=1.8��10-10��Ksp��AgI��=1.0��10-16�����������AgCl��AgI�ı�����Һ����Һ��ϣ��������м���һ������AgNO3���壬����˵����ȷ���ǣ�������
| A������Һ��ϣ�AgCl��AgI������ |
| B����AgI��Һ����AgNO3��c��Ag+������KSp��AgI��Ҳ���� |
| C����AgNO3������AgCl��AgI���ɳ���������AgClΪ�� |
| D����ȡ0.1435��AgCl�������100mLˮ����������仯����c��Cl-��Ϊ0.01mol/L |
A�����͵�AgCl��Һ�У�c��Ag+��=c��Cl-��=1.342��10-5��
���͵�AgI��Һ�У�c��Ag+��=c��I-��=1.0��10-8��
�������Ϻ�Ũ�ȼ��룬c��Cl-��=6.71��10-6��c��I-��=5.0��10-9��c��Ag+��=6.71��10-6
���ԣ���Ϻ�AgI��Ȼ������AgCl��Ȼ����A����
B��Ksp���¶��йأ��¶Ȳ��䣬Ksp���䣬��B����
C������c��Cl-����c��I-������AgNO3����������������AgClΪ����C��ȷ��
D�������£��Ȼ������ܽ��Ϊ��1.342��10-5��143.5�T0.001.92��g����c��Cl-���T1.342��10-5mol/L��0.1435��AgClֻ�������ܽ⣬��c��Cl-���T1.342��10-5mol/L����D����
��ѡC��
���͵�AgI��Һ�У�c��Ag+��=c��I-��=1.0��10-8��
�������Ϻ�Ũ�ȼ��룬c��Cl-��=6.71��10-6��c��I-��=5.0��10-9��c��Ag+��=6.71��10-6
���ԣ���Ϻ�AgI��Ȼ������AgCl��Ȼ����A����
B��Ksp���¶��йأ��¶Ȳ��䣬Ksp���䣬��B����
C������c��Cl-����c��I-������AgNO3����������������AgClΪ����C��ȷ��
D�������£��Ȼ������ܽ��Ϊ��1.342��10-5��143.5�T0.001.92��g����c��Cl-���T1.342��10-5mol/L��0.1435��AgClֻ�������ܽ⣬��c��Cl-���T1.342��10-5mol/L����D����
��ѡC��

��ϰ��ϵ�д�
�����Ŀ
��������ʵ���ó��Ľ��ۻ�����Ľ�����ȷ���ǣ� ��
|
|
ʵ����ʵ |
���ۻ���� |
|
�� |
��40 g NaOH����1 L����ˮ�� |
�� ��Һ�����ʵ���������Ϊ3.84%�������ʵ���Ũ��Ϊ1molL-1 |
|
�� |
��ʢ��1mL 0.1mol��L-1 AgNO3��Һ���Թ��еμ�0.1molL-1 NaCl��Һ���������г������ɣ��������е�0.1molL-1 KI��Һ����ɫ����ת��Ϊ��ɫ������ |
������ Ksp��AgCl��< Ksp��AgI�� |
|
�� |
ij��NaX��ҺpH > 7 |
��HX���� |
|
�� |
��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ |
˵����Һ��һ������Fe2+ |
|
�� |
��ʢ��Ũ������Թ��зֱ����AlƬ�� CuƬ��ǰ��û�����������߷�Ӧ���ң�������������ɫ���� |
��ԭ��Al < Cu |
|
�� |
BaSO4��ˮ��Һ�����Ժܲ� |
BaSO4��������� |
A���٢ܢ� B���ۢܢ� C���ۢ� D���ڢܢ�
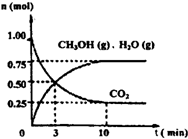 ��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���
��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���