��Ŀ����
ijͬѧ��a mol/L������KMnO4��Һ�ⶨ���ᾧ�壨H2C2O4?xH2O���е�xֵ��
��1����ȡW g���ᾧ�壬���250.00mL��ˮ��Һ��
�������������б����õ���2�ֶ���������______��______��
��������Ҫ�����У�����-����--______ת��--______����һҡ�ȣ�
��2����ȡ��õ�25.00mL������Һ������ƿ�У���a mol/L������KMnO4��Һ���еζ�����ȥV mL��
����ȡ25.00mL������Һ��������______��
����ƽ���ӷ���ʽ��______MnO4-+______H2C2O4+______H+--______Mn2++______CO2��+______H2O
�۵ζ��ﵽ�յ�ı�־��______��
��3�����������ݿɵ�x=______��
��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�͵���______
A����ȡW g���ᾧ��ʱ�������ᾧ�����������ƽ����
B����ƿ������ˮϴ����δ����
C���ζ��յ����ʱ���ӵζ��̶ܿ�
D��ʢKMnO4��Һ����ʽ�ζ��ܼ��첿�������ݣ��ζ���������ʧ��
�⣺��1��������ȷŨ�ȵIJ�����Һ������Ҫ��ʵ��������Ҫ��������ƽ�������룩���ձ���ҩ�ס�250mL����ƿ����ͷ�ιܡ��������ȣ�
�ʴ�Ϊ��250mL����ƿ��������ƽ��
��������Ҫ�����У����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ��ʴ�Ϊ���ܽ⣻ϴ�ӣ�
��2������ȡ25.00mL������Һ����ȷ��Ϊ0.01mL������ʹ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ����ʴ�Ϊ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ���
�ڷ�Ӧ��MnO4-��Mn2+��MnԪ�صĻ��ϼ���+7����Ϊ+2���ܹ�����5�ۣ�H2C2O4��CO2��CԪ�صĻ��ϼ���+3����Ϊ+4���ܹ�����2�ۣ����ϼ�������С������Ϊ10����MnO4-ϵ��Ϊ2����H2C2O4ϵ��Ϊ5��������Ԫ���غ������غ���ƽ�������ʵ�ϵ������ƽ��Ϊ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2��5��6��2��10��8��
�۲��ᷴӦ��ϣ��������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��˵���ζ��ﵽ�յ㣻
�ʴ�Ϊ���������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��3���ζ�25mL������Һ��Ҫ������ص����ʵ���Ϊa mol/L��V��10-3L=aV��10-3mol�����ݹ�ϵʽ2MnO4-��5H2C2O4����25.00mL������Һ�в�������ʵ���Ϊ2.5aV��10-3mol������Wg���ᾧ������ʵ���Ϊ2.5aV��10-3mol��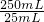 =0.025aVmol������
=0.025aVmol������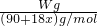 =0.025aVmol�����x=
=0.025aVmol�����x=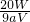 -5��
-5��
�ʴ�Ϊ��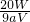 -5��
-5��
��4���ɣ�3���м����֪x=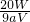 -5��
-5��
A����ȡW g���ᾧ��ʱ�������ᾧ�����������ƽ���̣�����ʹ�����룬�������ᾧ���������Ӱ�죬�Բⶨx��ֵ��Ӱ�죬��ʹ�����룬�����IJ��ᾧ�������ƫС�����ƵIJ�����Һ��Ũ��ƫС�����ĵĸ��������Һ�����ƫС������x��ֵƫ��A�����ϣ�
B����ƿ������ˮϴ����δ�����Ӱ����ƿ�ڲ�������ʵ�������Ӱ����������Һ���������x��ֵ��Ӱ�죬��B�����ϣ�
C���ζ��յ����ʱ���ӵζ��̶ܿȣ����������Һ����Ķ���ƫ������xֵƫ�ͣ���C���ϣ�
D��ʢKMnO4��Һ����ʽ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�����¸��������Һ����Ķ���ƫ������xֵƫ�ͣ���D���ϣ�
��ѡCD��
��������1��������ȷŨ�ȵIJ�����Һ������Ҫ��ʵ��������Ҫ��������ƽ�������룩���ձ���ҩ�ס�250mL����ƿ����ͷ�ιܡ��������ȣ�
��������Ҫ�����У����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��2������ȡ25.00mL������Һ����ȷ��Ϊ0.01mL������ʹ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ���
�ڷ�Ӧ��MnO4-��Mn2+��MnԪ�صĻ��ϼ���+7����Ϊ+2���ܹ�����5�ۣ�H2C2O4��CO2��CԪ�صĻ��ϼ���+3����Ϊ+4���ܹ�����2�ۣ����ϼ�������С������Ϊ10����MnO4-ϵ��Ϊ2����H2C2O4ϵ��Ϊ5��������Ԫ���غ������غ���ƽ�������ʵ�ϵ����
�۲��ᷴӦ��ϣ��������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��˵���ζ��ﵽ�յ㣻
��3�����ݹ�ϵʽ2MnO4-��5H2C2O4����25.00mL������Һ�в�������ʵ�������������Wg���ᾧ������ʵ������ٸ���n= ������ᾧ����x��ֵ��
������ᾧ����x��ֵ��
��4���ɣ�3���м����֪x=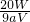 -5���ݴ˽��ѡ�������
-5���ݴ˽��ѡ�������
��������Ҫ����������ɵIJⶨ����ʵ��ԭ�������⡢��Һ���ơ�������ԭ�ζ���ʵ��������ʵ�����Ĵ��������ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������
�ʴ�Ϊ��250mL����ƿ��������ƽ��
��������Ҫ�����У����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ��ʴ�Ϊ���ܽ⣻ϴ�ӣ�
��2������ȡ25.00mL������Һ����ȷ��Ϊ0.01mL������ʹ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ����ʴ�Ϊ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ���
�ڷ�Ӧ��MnO4-��Mn2+��MnԪ�صĻ��ϼ���+7����Ϊ+2���ܹ�����5�ۣ�H2C2O4��CO2��CԪ�صĻ��ϼ���+3����Ϊ+4���ܹ�����2�ۣ����ϼ�������С������Ϊ10����MnO4-ϵ��Ϊ2����H2C2O4ϵ��Ϊ5��������Ԫ���غ������غ���ƽ�������ʵ�ϵ������ƽ��Ϊ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2��5��6��2��10��8��
�۲��ᷴӦ��ϣ��������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��˵���ζ��ﵽ�յ㣻
�ʴ�Ϊ���������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��3���ζ�25mL������Һ��Ҫ������ص����ʵ���Ϊa mol/L��V��10-3L=aV��10-3mol�����ݹ�ϵʽ2MnO4-��5H2C2O4����25.00mL������Һ�в�������ʵ���Ϊ2.5aV��10-3mol������Wg���ᾧ������ʵ���Ϊ2.5aV��10-3mol��
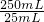 =0.025aVmol������
=0.025aVmol������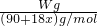 =0.025aVmol�����x=
=0.025aVmol�����x=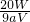 -5��
-5���ʴ�Ϊ��
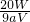 -5��
-5����4���ɣ�3���м����֪x=
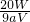 -5��
-5��A����ȡW g���ᾧ��ʱ�������ᾧ�����������ƽ���̣�����ʹ�����룬�������ᾧ���������Ӱ�죬�Բⶨx��ֵ��Ӱ�죬��ʹ�����룬�����IJ��ᾧ�������ƫС�����ƵIJ�����Һ��Ũ��ƫС�����ĵĸ��������Һ�����ƫС������x��ֵƫ��A�����ϣ�
B����ƿ������ˮϴ����δ�����Ӱ����ƿ�ڲ�������ʵ�������Ӱ����������Һ���������x��ֵ��Ӱ�죬��B�����ϣ�
C���ζ��յ����ʱ���ӵζ��̶ܿȣ����������Һ����Ķ���ƫ������xֵƫ�ͣ���C���ϣ�
D��ʢKMnO4��Һ����ʽ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�����¸��������Һ����Ķ���ƫ������xֵƫ�ͣ���D���ϣ�
��ѡCD��
��������1��������ȷŨ�ȵIJ�����Һ������Ҫ��ʵ��������Ҫ��������ƽ�������룩���ձ���ҩ�ס�250mL����ƿ����ͷ�ιܡ��������ȣ�
��������Ҫ�����У����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��2������ȡ25.00mL������Һ����ȷ��Ϊ0.01mL������ʹ��25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ���
�ڷ�Ӧ��MnO4-��Mn2+��MnԪ�صĻ��ϼ���+7����Ϊ+2���ܹ�����5�ۣ�H2C2O4��CO2��CԪ�صĻ��ϼ���+3����Ϊ+4���ܹ�����2�ۣ����ϼ�������С������Ϊ10����MnO4-ϵ��Ϊ2����H2C2O4ϵ��Ϊ5��������Ԫ���غ������غ���ƽ�������ʵ�ϵ����
�۲��ᷴӦ��ϣ��������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��˵���ζ��ﵽ�յ㣻
��3�����ݹ�ϵʽ2MnO4-��5H2C2O4����25.00mL������Һ�в�������ʵ�������������Wg���ᾧ������ʵ������ٸ���n=
 ������ᾧ����x��ֵ��
������ᾧ����x��ֵ����4���ɣ�3���м����֪x=
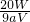 -5���ݴ˽��ѡ�������
-5���ݴ˽��ѡ���������������Ҫ����������ɵIJⶨ����ʵ��ԭ�������⡢��Һ���ơ�������ԭ�ζ���ʵ��������ʵ�����Ĵ��������ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
�� ��1����Ҫ��ȡŨ���� ������ mL��
��2������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ�����ǣ�������w.w.^w.k.&s.5*u.c.#om��.��.��.Դ.��
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶ȸĿ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
������ �� ������ �ȡ�
��4����������Һ��ת��������ƿʱ����������������������ҺŨ�� ������ 0.9 mol��L��1������ڡ��������ڡ���С�ڡ�����ͬ����
��5��ȡ�����Ƶ�ϡ����100 mL����һ��������п��ַ�Ӧ��пȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.448 L����μӷ�Ӧ��п������Ϊ ������ g���跴Ӧ����Һ�������Ϊ100 mL ����Ӧ����Һ��H�������ʵ���Ũ��Ϊ ������ mol/L��
��10�֣�ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
����1����Ҫ��ȡŨ���� ������ mL(ȷ��С�����һλ)��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ���200mL����ƿ�⣬�������õ��������� ������ �� ������ �ȡ�
��3������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ������( )
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
������ƿδ���T����������Һ����������ҺŨ�� ���� 0.9 mol��L-1������ڡ��������ڡ���С�ڡ�����ͬ����������ʱ���ӿ̶��ߣ���������ҺŨ�� ���� 0.9 mol��L-1��