��Ŀ����
(1)��Ũ�����з���ͭƬ���ٿ�ʼ��Ӧ�Ļ�ѧ����ʽΪ__________________________________��
����ͭ��ʣ�࣬��Ӧ��Ҫ����ʱ�ķ�Ӧ����ʽ��______________________________��
�۵ȷ�Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ����
_____________________________________________________________________��
(2)��100 mL������У�c(HNO3)=0.4 mol��L-1��c(H2SO4)=0.2 mol��L-1�������м���2.56 gͭ�ۣ��ȣ��ȳ�ַ�Ӧ����Һ��Cu2+�����ʵ���Ũ��Ϊ__________________________��
(3)14 gͭ���Ͻ���һ����ijŨ�ȵ�������Һ��Ӧ�����ų���������1.12 L(��״����)�������ͨ��ˮ�У�ǡ��ȫ����ˮ���գ���Ͻ���ͭ��������_________________g��
������(1)��ʼʱͭ��Ũ���ᷢ����Ӧ��Cu+4HNO3====Cu(NO3)2+2NO2��+2H2O������ʱ��ͭ��ϡHNO3��Ӧ������ʽΪ��3Cu+8HNO3====3Cu(NO3)2+2NO��+4H2O����Ӧֹͣ���ټ���ϡH2SO4��H+��![]() ����������������������3Cu+2
����������������������3Cu+2![]() +8H+====3Cu2++2NO��+4H2O��
+8H+====3Cu2++2NO��+4H2O��
(2)�⣺n(Cu)=2.56 g/64 g��mol-1=0.04 mol��n(H+)=0.4 mol��L-1��0.1 L+0.2 mol��L-1��0.1 L��2=0.08 mol��n(![]() )=0.4 mol��L-1��0.1 L=0.04 mol
)=0.4 mol��L-1��0.1 L=0.04 mol
���ݣ�3Cu+2![]() +8H+====3Cu2++2NO��+4H2O֪����Һ��n(H+)����������H+�����ʵ������㡣����n(Cu2+)=0.08 mol��
+8H+====3Cu2++2NO��+4H2O֪����Һ��n(H+)����������H+�����ʵ������㡣����n(Cu2+)=0.08 mol��![]() =0.03 mol����c(Cu2+)=0.03 mol/0.1 L=0.3 mol��L-1
=0.03 mol����c(Cu2+)=0.03 mol/0.1 L=0.3 mol��L-1
(3)�������⣬�䷴Ӧ����Ϊ��
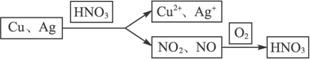
��������������14 g Cu��Agʧȥ����������0.5 mol O2�õ��ĵ�������
��Cu��Ag�����ʵ����ֱ�Ϊx��y��
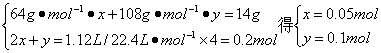
ͭ������Ϊ��m(Cu)=64 g��mol-1��0.05 mol=3.2 g
�𰸣�(1)��Cu+4HNO3(Ũ)====Cu(NO3)2+2NO2��+2H2O
��3Cu+8HNO3(ϡ)====3Cu(NO3)2+2NO��+4H2O
��H+��![]() �γ���ϡHNO3��Һ�����������ͭ��Ӧ����NO����
�γ���ϡHNO3��Һ�����������ͭ��Ӧ����NO����
(2)0.3 mol��L-1 (3)3.2

 ��У����ϵ�д�
��У����ϵ�д�