��Ŀ����
��֪25��ʱ�й�����ĵ���ƽ�ⳣ�����������й�˵����ȷ���ǣ�������
|
������A��������ĵ��볣���Ƚ����Ե�ǿ��������������������Ӷ�Ӧ����Խ������ˮ��̶�Խ����������
B�������Һ�е���غ��������c��Na+����c��CN-����������Һ�����Կ�֪c��H+����c��OH-����
C������������μ�ˮ����̶�һֱ������pHҲ�����������ӽ�������Һ��pH��
D�����õ���غ���������
B�������Һ�е���غ��������c��Na+����c��CN-����������Һ�����Կ�֪c��H+����c��OH-����
C������������μ�ˮ����̶�һֱ������pHҲ�����������ӽ�������Һ��pH��
D�����õ���غ���������
����⣺A���ɵ��볣��Ka�Ĺ�ϵ��֪��1.8��10-5��4.9��10-10��5.6��10-11��������CH3COOH��HCN��HCO3-����Ȼ��Ũ��ʱNa2CO3��ˮ��̶��������Һ��pH���������ʵ���Ũ�ȵĸ���ҺpH��ϵΪpH��Na2CO3����pH��NaCN����pH��CH3COONa������A��ȷ��
B����Һ�еĵ���غ㣺C��H+��+c��Na+��=c��CN-��+C��OH-����0.1mol?L-1NaCN��Һ��pH��7����֪c��H+����c��OH-������Һ�ʼ��ԣ���c��Na+����c��CN-����B����
C���ȱ���������μ�ˮ���������������Ũ����������������ˮ��������Ũ�ȱ�С�������Լ�С�����ڼ�ˮ�Ĺ����е���̶ȡ�pHһֱ������C����
D������Һ���Ե��ԣ������������Ӵ��ĵ���������������Ӵ��ĸ������������c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-������D����
��ѡA��
B����Һ�еĵ���غ㣺C��H+��+c��Na+��=c��CN-��+C��OH-����0.1mol?L-1NaCN��Һ��pH��7����֪c��H+����c��OH-������Һ�ʼ��ԣ���c��Na+����c��CN-����B����
C���ȱ���������μ�ˮ���������������Ũ����������������ˮ��������Ũ�ȱ�С�������Լ�С�����ڼ�ˮ�Ĺ����е���̶ȡ�pHһֱ������C����
D������Һ���Ե��ԣ������������Ӵ��ĵ���������������Ӵ��ĸ������������c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-������D����
��ѡA��
���������⿼������ˮ�⡢������ʵĵ��뼰��Һ������Ũ�ȵĹ�ϵ����ȷ��Һ�е����ʼ�����ǿ���ıȽϡ�����غ��ǽ����Ĺؼ���A��ѧ�������״��㣮

��ϰ��ϵ�д�
�����Ŀ
��֪25��ʱ�й�����ĵ���ƽ�ⳣ����
|
��֪25��ʱ�й�����ĵ���ƽ�ⳣ����
|
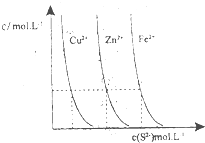 �����ǶԻ�ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺
�����ǶԻ�ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺