��Ŀ����
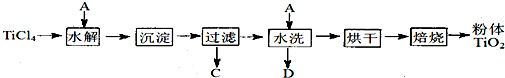
���Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2��xH2O�������ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2��
����NaOH�ⶨ��Һ��Ũ�ȣ�ȷ����1.000 gNaOH�������������Ƴ�250 mL��Һ��ȷ��ȡ25.00 mL��Һװ�ڼ�ʽ�ζ��ܣ��μ�2�η�̪��ָʾ��������Һװ����ʽ�ζ����У�����Һ���ų����ݺ���Һ�İ�Һ��պ��ڡ�0���̶ȣ��ζ�NaOH��Һ���ﵽ�յ��¼������ʵ���ظ�3�Σ���¼���±���

��ش��������⣺
(1)TiCl4ˮ������TiO2��xH2O�Ļ�ѧ����ʽΪ________��
(2)���Ƴ�250 mL��Һʹ�õ�������________��ָʾ��������________��
(3)�ζ��յ��������________��
(4)��Һ�����ʵ����ʵ���Ũ��Ϊ________��
(5)���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�Բⶨ���________��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)
������
|
����(1)TiCl4��(x��2)H2O ����(2)250 mL����ƿ(1�֣������250 mL����������)������(1��) ����(3)dz��ɫ��Һ��Ϊ��ɫ��30���ڲ���ɫ(2�֣���Ҫ���1��) ����(4)0.1250 mol��L��1(4�֣�������4λ��Ч������) ����(5)ƫ��(2��) |


 TiO2?xH2O��+4HCl
TiO2?xH2O��+4HCl