��Ŀ����
����Ŀ���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��N2(g)+3H2(g)![]() 2NH3(g) H����92.2 kJ��mol��1 ���ݴ˻ش��������⣺
2NH3(g) H����92.2 kJ��mol��1 ���ݴ˻ش��������⣺
��1����ij�¶��£����� 10 mol N2 �� 30 mol H2�������Ϊ 10 L ���ܱ������ڣ���Ӧ�ﵽƽ��״̬ʱ����û�������а����������Ϊ 20��������¶��·�Ӧ��K=_______ (���÷�����ʾ)��
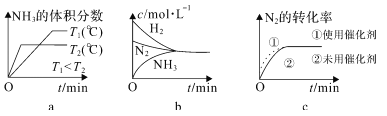
�ڶ��ںϳɰ���Ӧ���ԣ������й�ͼ��һ����ȷ����______��ѡ����ţ���
���ڼ�����Һ��ͨ����ⷨ����ʵ���� N2 ��ȡ NH3��2N2+6H2O![]() 4NH3+3O2�������ĵ缫��Ӧʽ��_______________��
4NH3+3O2�������ĵ缫��Ӧʽ��_______________��
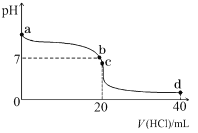
��2�������£����� 0.1 mol��L��1 ������� 20 mL 0.1 mol��L��1 ��ˮ�У���Һ pH �������������ı仯��������ͼ��ʾ��
��NH3��H2O �ĵ��뷽��ʽ��_______________��
��b ����ʾ��Һ�е�������_______________��
��c ����ʾ��Һ�У�����Ũ�ȴӴ�С�Ĺ�ϵΪ_______________��
�ܳ����£����� amol/LNH3��H2O �������� bmol/L �������ϣ���ַ�Ӧ����Һ������(�����ǰ�ˮ������Ļӷ�)������¶��� NH3��H2O �ĵ��볣��Ka=___________���ú� a �� b �Ĵ���ʽ��ʾ��
���𰸡� 1/12 ac N2+6e-+6H2O= 2NH3+6OH- NH3��H2O![]() NH4+��OH�C NH4Cl��NH3��H2O c(Cl�C) ��c (NH4+)��c (H+)��c (OH�C) 10-7b/(a-b)
NH4+��OH�C NH4Cl��NH3��H2O c(Cl�C) ��c (NH4+)��c (H+)��c (OH�C) 10-7b/(a-b)
����������1���� N2��g��+3H2��g��2NH3��g��
��ʼŨ�ȣ�mol/L�� 1 3 0
ת��Ũ�ȣ�mol/L�� x 3x 2x
ƽ��Ũ�ȣ�mol/L��1-x 3-3x 2x
ƽ���������а������������Ϊ20%����![]() ��100%=20%�����x=
��100%=20%�����x=![]() ��ƽ�ⳣ��K=
��ƽ�ⳣ��K=![]() =
=![]() ����a���ȳ��ֹյ���ȴﵽƽ�⣬��T2���¶ȸߣ�ƽ�������ƶ�����ͼ��һ�£�ѡ��a��ȷ��b��ƽ��ʱŨ�Ȳ�һ����ȣ�ƽ��Ũ��ȡ����ʼŨ�Ⱥ�ת���ʣ�ͼ���ʾ����ѡ��b����c��ʹ�ô����ӿ췴Ӧ���ʣ���ƽ����Ӱ�죬ƽ��ʱ���ʵ�Ũ�Ȳ��䣬��ͼ��һ�£�ѡ��c��ȷ����ѡac��������N2�õ����ӣ����ϼ����ߣ�������ԭ��Ӧ������OH-ʧȥ���ӷ���������Ӧ��������Ӧ��N2+6e-+6H2O=2NH3+6OH-����2����һˮ�ϰ���������ʣ���ˮ��Һ��ֻ�в��ֵ��룬��������������Ӻ�笠����ӣ�һˮ�ϰ��ĵ��뷽��ʽΪ��NH3��H2O
����a���ȳ��ֹյ���ȴﵽƽ�⣬��T2���¶ȸߣ�ƽ�������ƶ�����ͼ��һ�£�ѡ��a��ȷ��b��ƽ��ʱŨ�Ȳ�һ����ȣ�ƽ��Ũ��ȡ����ʼŨ�Ⱥ�ת���ʣ�ͼ���ʾ����ѡ��b����c��ʹ�ô����ӿ췴Ӧ���ʣ���ƽ����Ӱ�죬ƽ��ʱ���ʵ�Ũ�Ȳ��䣬��ͼ��һ�£�ѡ��c��ȷ����ѡac��������N2�õ����ӣ����ϼ����ߣ�������ԭ��Ӧ������OH-ʧȥ���ӷ���������Ӧ��������Ӧ��N2+6e-+6H2O=2NH3+6OH-����2����һˮ�ϰ���������ʣ���ˮ��Һ��ֻ�в��ֵ��룬��������������Ӻ�笠����ӣ�һˮ�ϰ��ĵ��뷽��ʽΪ��NH3��H2O![]() NH4+��OH�C����b����Һ�����ԣ���c��H+��=c��OH-�����Ȼ����ǿ�������Σ���ˮ��Һ�����ԣ�Ҫʹ��ˮ��Һ�����ԣ���ˮӦ����������������ΪNH4Cl��NH3��H2O����c����Һ�����ԣ�c ��H+����c ��OH-��������Һ�ĵ���غ�c��Cl-��+c��OH-��=c��NH4+��+c��H+����֪c ��Cl-����c ��NH4+������Һ������Ϊ笠�����ˮ��£���Ũ�ȴ�С˳��Ϊc ��Cl-����c ��NH4+����c ��H+����c ��OH-��������a molL-1�İ�ˮ��b molL-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH-��=1��10-7mol/L��
NH4+��OH�C����b����Һ�����ԣ���c��H+��=c��OH-�����Ȼ����ǿ�������Σ���ˮ��Һ�����ԣ�Ҫʹ��ˮ��Һ�����ԣ���ˮӦ����������������ΪNH4Cl��NH3��H2O����c����Һ�����ԣ�c ��H+����c ��OH-��������Һ�ĵ���غ�c��Cl-��+c��OH-��=c��NH4+��+c��H+����֪c ��Cl-����c ��NH4+������Һ������Ϊ笠�����ˮ��£���Ũ�ȴ�С˳��Ϊc ��Cl-����c ��NH4+����c ��H+����c ��OH-��������a molL-1�İ�ˮ��b molL-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH-��=1��10-7mol/L��
��Һ��c��NH4+��=c��Cl-��=![]() mol/L����Ϻ�Ӧǰc��NH3H2O��=
mol/L����Ϻ�Ӧǰc��NH3H2O��=![]() mol/L����Ӧ��c��NH3H2O��=��
mol/L����Ӧ��c��NH3H2O��=��![]() -
-![]() ��mol/L��Kb=
��mol/L��Kb= =
= =10-7
=10-7![]() ��
��