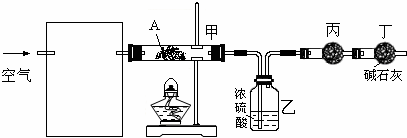
��Ŀ����
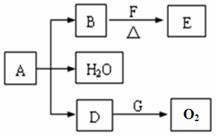
A��B��D��E��F��GΪ������Ԫ�أ���ԭ���������ε�����A��Fͬ���壬E��Gͬ���塣A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ���F�������ǽ���Ԫ�ػ���ʱ���γ����Ӽ�����F+������E2-���Ӻ�������Ų���ͬ��������Ԫ����ɵ�����BE��D2������ͬ�ĵ���������ش��������⣺(1)Fλ��___________���ڣ�____________�壻
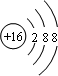
(2)G�����ӽṹʾ��ͼ__________________________________��
(3)�õ���ʽ��ʾD���γɹ���________________________________��
(4)��A��E��F����Ԫ���γɵĻ�����Ļ�ѧʽΪ__________�����еĻ�ѧ����__________(��д������ѧ������)������____________���壻
(5)B�������___________����A��B��ɵĻ������У���A����ߵ����ʵĻ�ѧʽ��__________����B����ߵ����ʵĻ�ѧʽ��____________(д������)����D2ʽ����ȵ����ʵĻ�ѧʽ��__________�����еĻ�ѧ����__________(��д������ѧ������)������__________���壬��___________�Թ��õ��Ӷԡ�
(1)3 ��A
(2)![]() (3)
(3) ![]()
(4)NaOH ���Ӽ��ͼ��Թ��ۼ� ���Ӿ���
(5)+4 CH

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ

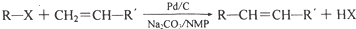
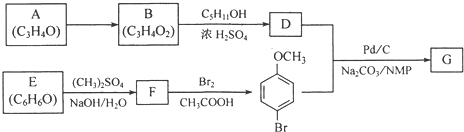

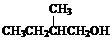
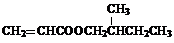 +H2O
+H2O
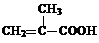
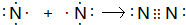

 CH3CHO+H2O+Cu
CH3CHO+H2O+Cu