��Ŀ����
ij����M��һ��þ��������ϣ���ȡ84g M�ڸ��������¼��ȷֽ⣬�õ�0.5mol��̬������A��ֻ���ж��ֶ�����Ԫ�أ���44.8L����B���������Ϊ��״���£�����������ʹʪ��ĺ�ɫʯ����ֽ��������̬������A������ˮ��Ӧ����������B������
����B������ת����ϵ��B
C
D
E��E��һ��ǿ�ᣮ��ش��������⣺
��1������A�Ļ�ѧʽΪ ������ ���壨����ӡ��������ӡ�����ԭ�ӡ��ȣ���
��2��д��M���·ֽ�Ļ�ѧ����ʽ ��
��3����C��D�Ļ�����ܽ��ڽӽ���ȵ�ˮ�У����ɵõ�һ������F��ˮ��Һ������F�ȴ���������ǿ���ܲ��ȶ���ͨ�����������ֽ⣮Ҫ�Ƶ�F��Һ���������䶳��������Ũ��Һ�м����ͨ��ij�����ʣ��������ʲ��ʺ�ʹ�õ��� ������ţ���
a������ b��������̼ c��ϡ���� d����������
��4���ַ���һ�ֻ��Ժ�ǿ�����ӻ�����G�������ΪNH5������ʽΪ ��NH5��ˮ��Ӧ�ܷ����H2 ����ܡ��롰���������� ��
��5������9.6gþ�뼫ϡ��E��Һ��ַ�Ӧ���������������������NaOH��Һ���ȣ�����B���壨��״���£� L��
����B������ת����ϵ��B
| X |
| X |
| H2O |
��1������A�Ļ�ѧʽΪ
��2��д��M���·ֽ�Ļ�ѧ����ʽ
��3����C��D�Ļ�����ܽ��ڽӽ���ȵ�ˮ�У����ɵõ�һ������F��ˮ��Һ������F�ȴ���������ǿ���ܲ��ȶ���ͨ�����������ֽ⣮Ҫ�Ƶ�F��Һ���������䶳��������Ũ��Һ�м����ͨ��ij�����ʣ��������ʲ��ʺ�ʹ�õ���
a������ b��������̼ c��ϡ���� d����������
��4���ַ���һ�ֻ��Ժ�ǿ�����ӻ�����G�������ΪNH5������ʽΪ
��5������9.6gþ�뼫ϡ��E��Һ��ַ�Ӧ���������������������NaOH��Һ���ȣ�����B���壨��״���£�
���㣺þ��������Ҫ������,������ƶ�
ר�⣺Ԫ�ؼ��仯����
������þ��������Ͼ��Ѿ�֪������MgԪ�أ�B��������ʹʪ��ĺ�ɫʯ����ֽ��������Ψһ�ļ�����NH3�����ݹ���A��ˮ��Ӧ��NH3���ɣ�����Ԫ���غ�A����þԪ�أ�Aֻ���ж��ֶ�����Ԫ������A���ɵ���þ����Ԫ����ɣ�0.5mol����Ϊ84g-2mol��17g?mol-1=50g�����Ƴ�A�Ļ�ѧʽ��Mg3N2��aM=1Mg3N2+4NH3����ѭ�����غ㶨�ɼ����ϼ۹��ɿ��Եó�MΪMg��NH2��2���������Ϸ�������������������⣮
���
�⣺þ��������Ͼ��Ѿ�֪������MgԪ�أ�B��������ʹʪ��ĺ�ɫʯ����ֽ��������Ψһ�ļ�����NH3�����ݹ���A��ˮ��Ӧ��NH3���ɣ�����Ԫ���غ�A����þԪ�أ�Aֻ���ж��ֶ�����Ԫ������A���ɵ���þ����Ԫ����ɣ�0.5mol����Ϊ84g-2mol��17g?mol-1=50g�����Ƴ�A�Ļ�ѧʽ��Mg3N2��aM=1Mg3N2+4NH3����ѭ�����غ㶨�ɼ����ϼ۹��ɿ��Եó�MΪMg��NH2��2��
��1��þ��������Ͼ��Ѿ�֪������MgԪ�أ�B��������ʹʪ��ĺ�ɫʯ����ֽ������NH3�����ݹ���A��ˮ��Ӧ��NH3���ɣ�0.5mol����Ϊ84g-2mol��17g?mol-1=50g�����Ƴ�A�Ļ�ѧʽ��Mg3N2������������ͨ�����Ӽ��γɵ����Ӿ��壬�ʴ�Ϊ��Mg3N2�����ӣ�
��2����84g M�ڸ��������¼��ȷֽ�õ�0.5mol��̬������A��ֻ���ж��ֶ�����Ԫ�أ���2mol����B���������Ϊ��״���£���aM=1Mg3N2+4NH3����ѭ�����غ㶨�ɼ����ϼ۹��ɿ��Եó�MΪMg��NH2��2����M���·ֽ�Ļ�ѧ����ʽΪ3Mg��NH2��2=Mg3N2+4NH3�����ʴ�Ϊ3Mg��NH2��2=Mg3N2+4NH3����
��3����ת��B
C
D
E��֪��C����NO��D��NO2��E�����ᣬNO2+NO+H2O=2HNO2��FΪ�����������������ԡ������Ժͻ�ԭ�ԣ�������ȴ���������ǿ����̼������Աȴ���������������ǿ��ԭ�ԣ��ʲ���ѡ��CO2��SO2���ʴ�Ϊ��bd��
��4��NH5�ĵ���ʽΪ��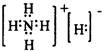 ��NH5�����и�һ�۵�����ǿ��ԭ�ԣ�Ҫ��ˮ����һ�۵��ⷢ�����з�Ӧ����������
��NH5�����и�һ�۵�����ǿ��ԭ�ԣ�Ҫ��ˮ����һ�۵��ⷢ�����з�Ӧ����������
�ʴ�Ϊ���ܣ�NH4H��-1�۵�H��H2O��+1�۵�H�������з�Ӧ��
��5�����ݵ�ʧ�����غ㣬�г���ϵʽΪ
4Mg��NH3
4��24g 22.4L
9.6g 2.24L
�ʴ�Ϊ��2.24L��
��1��þ��������Ͼ��Ѿ�֪������MgԪ�أ�B��������ʹʪ��ĺ�ɫʯ����ֽ������NH3�����ݹ���A��ˮ��Ӧ��NH3���ɣ�0.5mol����Ϊ84g-2mol��17g?mol-1=50g�����Ƴ�A�Ļ�ѧʽ��Mg3N2������������ͨ�����Ӽ��γɵ����Ӿ��壬�ʴ�Ϊ��Mg3N2�����ӣ�
��2����84g M�ڸ��������¼��ȷֽ�õ�0.5mol��̬������A��ֻ���ж��ֶ�����Ԫ�أ���2mol����B���������Ϊ��״���£���aM=1Mg3N2+4NH3����ѭ�����غ㶨�ɼ����ϼ۹��ɿ��Եó�MΪMg��NH2��2����M���·ֽ�Ļ�ѧ����ʽΪ3Mg��NH2��2=Mg3N2+4NH3�����ʴ�Ϊ3Mg��NH2��2=Mg3N2+4NH3����
��3����ת��B
| X |
| X |
| H2O |
��4��NH5�ĵ���ʽΪ��
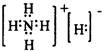 ��NH5�����и�һ�۵�����ǿ��ԭ�ԣ�Ҫ��ˮ����һ�۵��ⷢ�����з�Ӧ����������
��NH5�����и�һ�۵�����ǿ��ԭ�ԣ�Ҫ��ˮ����һ�۵��ⷢ�����з�Ӧ�����������ʴ�Ϊ���ܣ�NH4H��-1�۵�H��H2O��+1�۵�H�������з�Ӧ��
��5�����ݵ�ʧ�����غ㣬�г���ϵʽΪ
4Mg��NH3
4��24g 22.4L
9.6g 2.24L
�ʴ�Ϊ��2.24L��
���������⿼����ۺϣ���ȷ�ƶ�Ԫ�صĽⱾ��ؼ����������ʽṹ��������������ɣ��Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
A��1���������ӵ�ʵ������ԼΪ��
| ||
| B����NA����ԭ�ӵ������ڱ�״���µ����ԼΪ22.4L | ||
| C������������ˮ��Ӧ��ÿ������״����11.2L O2��ת��NA������ | ||
| D��1mol Na ԭ���к���NA������ |
����˵���У���ȷ���ǣ�������
| A��ԭ����ǽ�����ת��Ϊ��ѧ�ܵ�װ�� |
| B����ԭ����У������Ϸ���������Ӧ |
| C����ԭ����У����Ӵ�ԭ��ص��������� |
| D����ԭ����ڲ��������������������ƶ� |
���з�ɢϵ���ܷ��������������ǣ�������
| A������ | B���̡��ơ��� |
| C��NaCl��Һ | D��ţ�� |
���и�����Ͷ��ˮ�У���ٽ�ˮ�ĵ��������ʹ��Һ�����Ե��ǣ�������
| A��Al2��SO4��3 |
| B��Na3PO4 |
| C��CH3COOH |
| D��NaHSO4 |