��Ŀ����
4�������й�˵������ȷ���ǣ�������| A�� | 0.1 mol/L Na2SO3��Һ��c��Na+��=2c��SO32-��+c��HSO3-��+c��H2SO3�� | |
| B�� | 0.1 mol•L-1 NaHCO3��Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� | |
| C�� | �Ե��з�̪��CH3COONa��Һ���м��ȣ���Һ��ɫ����� | |
| D�� | ��pH=5.6��CH3COOH��CH3COONa�Ļ����Һ�У�c��Na+����c��CH3COO-�� |
���� A����������������Һ�е������غ��жϣ�
B��̼��������Һ�У�̼��������ӵĵ���̶�С����ˮ��̶ȣ���Һ��ʾ���ԣ���c��H+����c��OH-����c��H2CO3����c��CO32-����
C����������ӵ�ˮ��Ϊ���ȷ�Ӧ�������¶Ⱥ��������ӵ�ˮ��̶���������Һ������������Ũ����������ǿ��
D��pH=5.6�����ҺΪ���ԣ���c��H+����c��OH-������ϵ���غ��֪��c��Na+����c��CH3COO-����
��� �⣺A.0.1 mol/L Na2SO3��Һ�У����������غ�ɵã�c��Na+��=2c��SO32-��+2c��HSO3-��+2c��H2SO3������A����
B.0.1 mol•L-1 NaHCO3��Һ�У�HCO3-�ĵ���̶�С����ˮ��̶ȣ���Һ��ʾ���ԣ���c��H2CO3����c��CO32-������Һ����ȷ��Ũ�ȴ�СΪ��c��Na+����c��HCO3-����c��H2CO3����c��CO32-������B����
C���Ե��з�̪��CH3COONa��Һ���м��ȣ���������ӵ�ˮ��̶�������Һ������ǿ��������Һ��ɫ������C��ȷ��
D��CH3COOH��CH3COONa�Ļ����Һ��pH=5.6�����ҺΪ���ԣ���c��H+����c��OH-������ϵ���غ��֪��c��Na+����c��CH3COO-������D����
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע�������ε�ˮ��ԭ������Ӱ�죬��ȷ����غ㡢�����غ����ж�����Ũ�ȴ�С�е�Ӧ�÷�����������ؿ���ѧ���ķ���������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��Һ��Ca2+��Ŀ���� | B�� | c��Ca2+������ | ||
| C�� | ��Һ��c��OH-������ | D�� | ��Һ��OH-��Ŀ���� |
| A�� | 1��1 | B�� | 5��4 | C�� | 4��3 | D�� | 5��3 |

��֪����һ����ͬ�¶���H3BO3���ܽ��
| �¶ȡ棩 | 20 | 40 | 60 | 100 |
| �ܽ�ȣ�g�� | 5.0 | 8.7 | 14.8 | 40.2 |
| ���� | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 3.2 | 5.2 | 9.7 | 12.4 |
��2��������Һ�������ԣ�����H3BO3��Mg2+��SO42-��������Fe2+��Fe3+��Ca2+��Al3+�����ʣ������ӡ�ʱ�������Һ�����μ�������ĿH2O2��MgO�����Գ�ȥ����������ΪFe3+��Fe2+��Al3+��������ΪH2O2+2H++2Fe2+=2Fe3++2H2O�������ӷ���ʽ��ʾ����
��3������ȡ�����á��ȹ��ˡ���Ŀ��Ϊ��ֹ�¶��½�ʱH3BO3����Һ��������
��4����ĸҺ�������ڻ�������þ����֪����þ���ܽ�����¶ȱ仯��������ͼ��ʾ������Һ�ķе���ѹǿ��������ߣ�Ϊ�˴ӡ�ĸҺ���г�ֻ��� MgSO4��H2O��Ӧ��ȡ�Ĵ�ʩ�ǽ���ĸҺ������Ũ������ѹ���½ᾧ��

��5����֪25��ʱ�����ᣨ H3BO3����Һ�д�������ƽ�⣺HBO3��aq��+H2O��l��?[B��OH��4]-��aq��+H+��aq�� K=5.7��10-10��25��ʱ��0.7mol��L-1������Һ��c��H��+��2��10-5mol��L-1
��6����֪25��ʱ��
| ��ѧʽ | H2CO3 | CH3COOH |
| ���볣�� | K1=4.4��10-7 K2=4.7��10-11 | K=1.75��10-5 |
A��̼������Һ����������Һ���ܹ۲쵽�����ݲ���
B��̼������Һ���������Һ���ܹ۲쵽�����ݲ���
C����Ũ��̼����Һ��������Һ��pH��ǰ�ߣ�����
D����Ũ��̼������Һ�ʹ�������Һ��pH��ǰ�ߣ����ߣ�
| ʵ����� | ��ȡ��Ʒ������/g | ����Ba��OH��2��Һ�����/mL | ������ɳ���������/g |
| 1 | 0.858 | 500 | 1.379 |
| 2 | 1.716 | 500 | |
| 3 | 2.574 | 500 | 4.137 |
| 4 | 3.432 | 500 | 5.516 |
| 5 | 4.290 | 500 | 5.516 |
| 6 | 5.148 | 500 | 5.516 |
��1��д������NaHCO3����Ba��OH��2��Һ��Ӧ�����ӷ���ʽBa2++HCO3-+OH-�TBaCO3��+H2O��
��2����2��ʵ���в���������������2.758g��
��3����Ʒ��NaHCO3��K2CO3�����ʵ���֮����2��5��
��4��ԭBa��OH��2��Һ��Ũ��Ϊ0.056mol•L-1��
��5��������ȡ��3��ʵ��������Һ��1/10����ˮ���500mL��Һ����ϡ���Ժ���Һ��pHֵΪ12��
 3-������
3-������ 2��2��3-��������
2��2��3-��������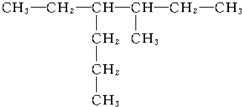 3-��-4-�һ�����
3-��-4-�һ����� 2��5-����-3-�һ�����
2��5-����-3-�һ����� 3��4-����-5-�һ�����
3��4-����-5-�һ����� 3-��-4-�һ����飮
3-��-4-�һ����飮


