��Ŀ����
��21�֣�ʵ����������80mL��1.5 mol/L��NaHCO3��Һ���Իش�
��1����ʵ�����ʹ�õIJ������� ��
��2�����ø���ҺʱӦ��ȡNaHCO3������Ϊ_____________________����3�����в���������������Һ���ʵ���Ũ�ȵ�Ӱ�죨��д��Ӱ�졢ƫ��ƫ�ͣ�
A�����ƹ�����δϴ���ձ��Ͳ�������
B������ƿʹ��֮ǰδ��ɣ�����������ˮ��
C������ʱ��������ƿ�Ŀ̶��ߣ�
D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦����__________
д�����з�Ӧ�����ӷ���ʽ��
A����NaHCO3��Һ�еμ�����
B����Ba(OH)2��Һ�еμ�����NaHCO3��Һ
C����ˮ�еμ�MgCl2��Һ
��1���ձ�������������ͷ�ιܡ�100ml����ƿ����2��12.6g����3�� A��ƫ��
B����Ӱ�� C��ƫ�� D����Ӱ��
��4�� A��HCO3-+H+=H2O+CO2�� B��HCO3-+Ba2++OH-=BaCO3��+H2O
C��2NH3��H2O+Mg2+=Mg(OH)2��+2NH4+
���������������1����ʵ�����ʹ�õIJ������У��ձ�������������ͷ�ιܡ�100ml����ƿ��
��2��Ҫ����80mL��1.5 mol/L��NaHCO3��Һ��ʵ���ϸ�������ƿ�Ĺ��Ҫ����100��������Һ���ʳ�ȡNaHCO3������Ϊ0.1��1.5��84= 12.6g����3��A�����ƹ�����δϴ���ձ��Ͳ��������ձ��Ͳ�������մ�����ʣ��������Ƶ���Һ��Ũ��ƫ�ͣ�B������ƿʹ��֮ǰδ��ɣ�����������ˮ����Ӱ�������Һ�����������Ӱ�죻C������ʱ��������ƿ�Ŀ̶��ߣ���Һ�����ƫС����Ũ��ƫ�ߣ�D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦������Һ���٣�����Ӱ��Ũ�ȡ���4��A����NaHCO3��Һ�еμ����̼�����Ʋ�������Ӻ�̼��������ӣ������������Ӻ������ӣ���Ӧ�����Ȼ��Ʋ�����ӣ������ӷ���ʽд�ɣ�HCO3-+H+=H2O+CO2����B����Ba(OH)2��Һ�еμ�����NaHCO3��Һ����Ӧ����̼�ᱵ���������������ƣ������ӷ���ʽд�ɣ�HCO3-+Ba2++OH-=BaCO3��+H2O��C����ˮ�еμ�MgCl2��Һ����Ӧ����������þ���������Ȼ�泥������ӷ���ʽд��2NH3��H2O+Mg2+=Mg(OH)2��+2NH4+��
���㣺����һ�����ʵ���Ũ�ȵ���Һ�������������ӷ���ʽ����д��

 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д����������ҹ�������н�ֹ����ʹ�ú�Ǧ���ͣ�����Ҫԭ����
| A���������ȼ��Ч�� | B���������ͳɱ� |
| C������Ǧ��Ⱦ���� | D��Ǧ��Դ��ȱ |
�������ೱ���ҹ�ʱ�з��������ೱ����ʱ����ˮ�е�ijЩС�������������ֳ��ʹˮ�ʺ졢�ϵ���ɫ�������������Σ��������˵������ȷ����
| A���ೱ��ˮ�帻Ӫ�����Ľ�� |
| B������ϴ�Ӽ��Ĺ㷺Ӧ�����ŷ��Ƿ����ೱ����Ҫԭ�� |
| C���ڷ�յĺ���������ೱ |
| D���ೱ�ķ������������ص���Ȼ���� |
15��)ʵ�������������ƹ�������1��0 mol/L��NaOH��Һ500 mL���ش��������⣺
��1�����Ҫ������ʵ�����Ҫʵ�鲽�裺
��__________________����__________________��
��__________________����__________________��
��__________________����__________________��
��2����������Ϊ������ƿ(���Ϊ________)��������ƽ������Ҫ��Щʵ������������ɸ�ʵ�飬��д����_________________________________________
��3�����в�����������Һ��Ũ���к�Ӱ�죿(��д��ĸ)
ƫ�����_______________��ƫС���� ����Ӱ����� ��
| A������ʱʹ������������� |
| B����NaOH����ֽ���ϳ��� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�� |
| D��������ƿ����Һʱ��������Һ�彦�� |
F������ʱ���ӿ̶���
G������ƿδ���T����������Һ
H�����ݺ����ƿ������ҡ�ȣ����ú���Һ�治���̶��ߣ��ټ�ˮ���̶���
��12�֣�ʵ�������ܶ�Ϊ1��18g/mL����������Ϊ36��5%Ũ��������250mLO��lmol/L��ϡ������Һ��ղ���ش��������⣺
��1��ʹ������ƿǰ������е�һ��������______________________________��
��2������250mL0��lmol/L����ϡ����Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________________________________��
| A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ���� |
| B������Ͳȷ��ȡ����Ũ�������__________mL���ز����������ձ��У��ټ�������ˮ(Լ30mL)���ò���������������ʹ���Ͼ��� |
| C��������ȴ�������ز�����ע��mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��?��û�н���A����Ũ��__________ ��������ʱ���ӿ̶��ߣ�Ũ��___________��
��8�֣�Ϊ��֤������Cl2 > Fe3�� > SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��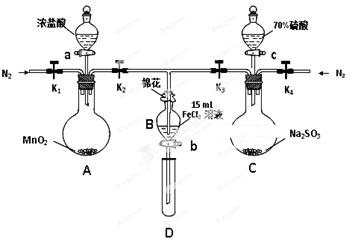
ʵ����̣�
I. ���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��. ����a���μ�һ������Ũ���ᣬ��A���ȡ�
��. ��B����Һ���ʱ��ֹͣ���ȣ��ر�K2��
��. ����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��. ��K3�ͻ���c������70%�����ᣬһ��ʱ���ر�K3��
��. �����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1�����̢��Ŀ���� ��
��2�����н������ҺΪ ��
��3��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��4��������ȡ��SO2ͨ�����Ը��������Һ��ʹ��Һ��ɫ�������ӷ���ʽΪ ��
��5���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬�������±���ʾ�����ǵļ����һ���ܹ�֤��������Cl2 > Fe3�� > SO2���� ����ס����ҡ�����������
| | ���̢�B��Һ�к��е����� | ���̢�B��Һ�к��е����� |
| �� | ��Fe3����Fe2�� | ��SO42�� |
| �� | ����Fe3������Fe2�� | ��SO42�� |
| �� | ��Fe3����Fe2�� | ��Fe2�� |

