��Ŀ����
Ϊ�о�CH3COOH��HA��MOH������Ե����ǿ����ij��ȤС�����������ʵ�飺�����£���pH=2����������ҺCH3COOH��HA��pH=12�ļ�MOH��Һ��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ1��ʾ�������������ݣ���ش��������⣺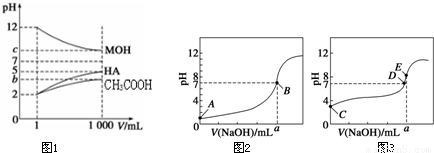
��1��HAΪ______�� ���ǿ������������ϡ�ͺ� HA��Һ��ˮ���������c��H+��______������Һ��ˮ�������c��H+��������ڡ��������ڡ���С�ڡ�����
��b+c=14����MOHΪ______��ǿ������������
��2��������0.10mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ��������______��
A��c��H+�� B��c��H+��/c��CH3COOH��
C��c��H+��?c��OH-�� D��c��OH-��/��H+��
��3�������£���0.10mol?L-1 NaOH��Һ�ֱ�ζ�20mL 0.10mol?L-1 HCl��Һ��20mL 0.10 mol?L-1 CH3COOH��Һ���õ������ζ����ߣ���ͼ��ʾ����ͼ�еζ�������Һ��������______���ͼ2����ͼ3������������E��pH��8��ԭ���ǣ������ӷ���ʽ��ʾ��______��
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ������Ũ���ɴ�С˳��Ϊ______��
���𰸡���������1��pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣻ϡ�ͺ� HA��ҺPH���ڴ�����Һ��PH����HA��Һ��c��H+��С�ڴ�����Һ��c��H+������Һ��c��H+��ԽС����ˮ�ĵ�������ƾ�ԽС��ˮ�����c��H+��Խ������ϡ�ͺ� HA��Һ��ˮ���������c��H+�����ڴ�����Һ��ˮ�������c��H+����b+c=14������Һ�е�c��H+������е�c��OH-����ȣ�˵�����ߵ���̶���ͬ���ʼ�Ϊ���
��2����Һ�д�������ƽ��CH3COOH?CH3COO-+H+����ˮƽ�����ƣ�c��H+�� ��С��c��OH-������Kw���䣻
��3�����������ᣬ���ڵ���ƽ�⣬��ҺPH�仯������CH3COOH��NaOHǡ�÷�Ӧʱ���ɵ�CH3COONaˮ�⣬��Һ�ʼ��ԣ�
��4��CH3COOH��CH3COONa�Ļ����Һ�����ԣ���CH3COOH�ĵ���Ϊ����
����⣺��1��pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣬�ݴ˿�ȷ��HAΪǿ�ϡ�ͺ� HA��ҺPH���ڴ�����Һ��PH����HA��Һ��c��H+��С�ڴ�����Һ��c��H+������Һ��c��H+��ԽС����ˮ�ĵ�������ƾ�ԽС��ˮ�����c��H+��Խ��b+c=14������Һ�е�c��H+������е�c��OH-����ȣ�˵�����ߵ���̶���ͬ���ʼ�Ϊ���
�ʴ�Ϊ��ǿ�����ڣ�����
��2����Һ�д�������ƽ��CH3COOH?CH3COO-+H+����ˮƽ�����ƣ�
A����ˮϡ��PH����c��H+�� ��С����A����
B�� =
= ��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��
��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��
C��c��H+��?c��OH-�� ֻ���¶��йأ���ˮ���䣬��C����
D����ˮϡ��PH����c��H+�� ��С��c��OH-�����ʶ��߱�ֵ����D��ȷ��
�ʴ�Ϊ��BD��
��3�����������ᣬ���ڵ���ƽ�⣬��ҺPH�仯��������PH�仯�����Ǵ��ᣬ��ͼ2�Ǵ���ģ���CH3COOH��NaOHǡ�÷�Ӧʱ���ɵ�CH3COONaˮ�⣬��Һ�ʼ��ԣ�����PH����7ˮ�ⷽ��Ϊ��CH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��ͼ2��CH3COO-+H2O?CH3COOH+OH-��
��4��CH3COOH��CH3COONa�Ļ����Һ�����ԣ���CH3COOH�ĵ���̶ȴ���CH3COONa��ˮ��̶�����c��CH3COO-����c��Na+������Һ�����ԣ�c��H+����c��OH-�������c��CH3COO-����c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
���������⿼����������ʵĵ���������ˮ�⣬������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ�һ�㣮
��2����Һ�д�������ƽ��CH3COOH?CH3COO-+H+����ˮƽ�����ƣ�c��H+�� ��С��c��OH-������Kw���䣻
��3�����������ᣬ���ڵ���ƽ�⣬��ҺPH�仯������CH3COOH��NaOHǡ�÷�Ӧʱ���ɵ�CH3COONaˮ�⣬��Һ�ʼ��ԣ�
��4��CH3COOH��CH3COONa�Ļ����Һ�����ԣ���CH3COOH�ĵ���Ϊ����
����⣺��1��pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣬�ݴ˿�ȷ��HAΪǿ�ϡ�ͺ� HA��ҺPH���ڴ�����Һ��PH����HA��Һ��c��H+��С�ڴ�����Һ��c��H+������Һ��c��H+��ԽС����ˮ�ĵ�������ƾ�ԽС��ˮ�����c��H+��Խ��b+c=14������Һ�е�c��H+������е�c��OH-����ȣ�˵�����ߵ���̶���ͬ���ʼ�Ϊ���
�ʴ�Ϊ��ǿ�����ڣ�����
��2����Һ�д�������ƽ��CH3COOH?CH3COO-+H+����ˮƽ�����ƣ�
A����ˮϡ��PH����c��H+�� ��С����A����
B��
 =
= ��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��
��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��C��c��H+��?c��OH-�� ֻ���¶��йأ���ˮ���䣬��C����
D����ˮϡ��PH����c��H+�� ��С��c��OH-�����ʶ��߱�ֵ����D��ȷ��
�ʴ�Ϊ��BD��
��3�����������ᣬ���ڵ���ƽ�⣬��ҺPH�仯��������PH�仯�����Ǵ��ᣬ��ͼ2�Ǵ���ģ���CH3COOH��NaOHǡ�÷�Ӧʱ���ɵ�CH3COONaˮ�⣬��Һ�ʼ��ԣ�����PH����7ˮ�ⷽ��Ϊ��CH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��ͼ2��CH3COO-+H2O?CH3COOH+OH-��
��4��CH3COOH��CH3COONa�Ļ����Һ�����ԣ���CH3COOH�ĵ���̶ȴ���CH3COONa��ˮ��̶�����c��CH3COO-����c��Na+������Һ�����ԣ�c��H+����c��OH-�������c��CH3COO-����c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
���������⿼����������ʵĵ���������ˮ�⣬������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ�һ�㣮

��ϰ��ϵ�д�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�
�����Ŀ
�ڴ��������������ͨ��������Ӧ�����飬��Ӧԭ�����£�
��CH3COOH+2H2��CH3CH2OH+H2O
��CH3CH2OH+H2��CH3CH3+H2O
��CH3COOH+3H2��CH3CH3+2H2O
�ڷ�Ӧ������������Ҵ�����������������Ϊ�о��ֱ��˼�Mo���⣩�Ͳ���Mo������Ni������������Ч�ܣ�ij�����ص��������£�
|
|
����ת���� |
δ��Ӧ�ĺ��������ﺬ�� |
||||
|
���� |
�Ҵ� |
|||||
|
�¶�/�� |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
|
240 |
87.2 |
21.8 |
0.53 |
3.24 |
1.03 |
0.14 |
|
260 |
89.1 |
27.2 |
0.45 |
3.02 |
0.99 |
0.19 |
����˵������ȷ���� �� ��
A�������٢ڢ�����Ӧ�����ڼӳɷ�Ӧ
B�������٢ڢ�����Ӧ�����ڻ�ԭ��Ӧ
C���������������Ӧ260���240���½��и�����
D��Mo16Ni6�����ܱ�Ni6��

 CH3COOH+OH-
CH3COOH+OH-






 ��
��
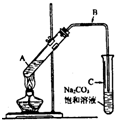 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O