��Ŀ����
���ݱ�����Ϣ�ش��������⡣
| Ԫ�� | Si | P | S | Cl |
| ���������� ��Ӧ������ | ���� | �������������ܷ�Ӧ | ���� | ���ջ��ȼʱ������ը������ |
��1��S��Ԫ�����ڱ��е�λ���� ��
��2�����ݱ�����Ϣ��֪��Si��P��S��Cl ����Ԫ�صĵķǽ�����������ǿ����ԭ�ӽṹ����ԭ��ͬ����Ԫ�ص��Ӳ�����ͬ���������ң� ��ԭ�Ӱ뾶��С���õ�����������ǿ��
��3��25��ʱ����������Ԫ�صĵ�����������Ӧ����l mol��̬�⻯��ķ�Ӧ�����£�
a��+34.3 kJ��mol-1 b��+9.3 kJ��mol-1 c��?20.6 kJ��mol-1 d��?92.3 kJ��mol-1
��д����̬���ף�P4����H2��Ӧ������̬�⻯����Ȼ�ѧ����ʽ ��
��4��̽��ͬ����Ԫ�����ʵ�һЩ��ͬ���ɣ���ѧϰ��ѧ����Ҫ����֮һ����֪����Se��������������Ԫ�أ��䲿����Ϣ��ͼ��

�������й�˵����ȷ���� ������ĸ����
a. ԭ�Ӱ뾶��Se��S��P b. �ȶ��ԣ�H2Se��H2S
c. ��Ϊ����H2Se��HCl�����Էǽ�����Se��Cl
d. SeO2����������������ռ���Һ��Ӧ
�����±����г���H2SeO3���ֲ�ͬ��ѧ���ʵ��Ʋ⣬������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | �����Ʋ� | ��ѧ����ʽ |
| 1 | ������ | H2SeO3��4HI=Se����2I2��3H2O |
| 2 | | |
| 3 | | |
��1����3����VIA��2���˵������������������
��3��P4��s��+6H2(g)=4PH3(g) ��H=+37.2 kJ��mol-1
��4����d
��1 ����Ԥ�� ��ѧ����ʽ 2 ��ԭ�� H2SeO3+Cl2+H2O=" " H2SeO4+2HCl 3 ���� H2SeO3+2NaOH=Na2 SeO3+2H2O.
���������������1������2���μ��𰸣���3����д����̬���ף�P4����H2��Ӧ������̬�⻯��Ļ�ѧ����ʽ��P4+6H2=4PH3��ͨ����ѧ����ʽ���Կ�����1mol P4��H2��Ӧ����4 mol PH3��������������������Ӧ����4 mol PH3ʱ����Ӧ�ų�������Ϊ��4��+9.3 kJ��mol-1=+37.2 kJ��mol-1 �������Ȼ�ѧ����ʽΪ��
P4��s��+6H2(g)=4PH3(g) ��H=+37.2 kJ��mol-1��4����a.����ͬ���ں�ͬ����Ԫ��ԭ�Ӱ뾶�ĵݱ���ɣ���֪��ȷ��ϵӦΪ��Se��P��S������b.ͬ����Ԫ����̬�⻯����ȶ������϶������μ���,��ȷ��ӦΪ: H2Se��H2S ������c.Ӧ��Ϊ����������Ӧ��ˮ��������ԣ�����H2SeO4��HClO4,��ǽ�����Se��Cl������d.����ͬ����Ԫ�ص����ʵ������ԣ����ѵ�֪d��ȷ��
��4���о�H2SeO3�Ļ�ѧ���ʿɴ�H2SO3�Ļ�ѧ���ʽ������ƣ��������л�ԭ�ԣ���������������Ӧ���ɴ˿��ƶ�H2SeO3Ҳ�л�ԭ�ԣ���ѧ����Ϊ�� H2SeO3+Cl2+H2O= H2SeO4+2HCl�������������ԣ���������Ӧ���������H2SeO3Ҳ�ɴ����ʣ���ѧ����ʽΪ��H2SeO3+2NaOH=Na2 SeO3+2H2O.
���㣺����Ԫ�����ڱ���Ԫ�������ɡ�ԭ�ӽṹ��ԭ�Ӱ뾶�Ĵ�С�Ƚϡ��Ȼ�ѧ���̵���д

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д���A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | A�ĵ������ܶ���С������ |
| B | B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| C | C��ԭ�����������������ڲ������������ |
| D | D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С |
| E | B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ� |
| F | FԪ���������������۵Ĵ�����Ϊ4 |
��2��D��E��F�ļ����Ӱ뾶�ɴ�С��˳����(ֱ���û�ѧʽ��ʾ) ��
��3����Fe��D������ɵĻ�����У���������F������������Ӧˮ�����ϡ��Һ������ȫ���ܽ⡣�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȡ���ԭ�������D���ʵ���������
Ϊ ��
��4��һ������ʯ������ͨ��һ������E���ʣ�����ǡ����ȫ��Ӧ���������������ֺ�EԪ�ص����ӣ������������ӵ����ʵ���(n)�뷴Ӧʱ��(t)��������ͼ��ʾ����ʱ��Ӧ�Ļ�ѧ����ʽΪ ��
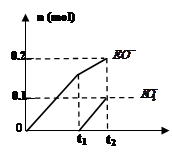
��5��A��B�γɵĻ�����BA���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƵĻ����д�������Ҵ���Ӧ�Ļ�ѧ����ʽ ��
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã�����Ӧ��ѧ����ش��������⣺
| ������ | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
��2���ܢݢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________�������ӷ��ţ����ۢߢ������������Ӧˮ�����������ǿ������˳��Ϊ____���ѧʽ����
��3���ݺ͢��γɻ�����ľ�������Ϊ__________��
��4����ЩԪ���γɵ��������У�������ˮ����������ǿ��������ǿ�Ӧ����___________���ѧʽ����д������ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽ__________��
��5��X��Y�ɢ٢ڢ��е����ֻ�����Ԫ����ɡ�X����Һ����С�մ�Ӧ����Y����X��������ϵ������ķ��ӣ�����Է�������Ϊ46����X������Ϊ ��д��X��Һ��С�մ�Ӧ�Ļ�ѧ����ʽΪ_______________��
X��R��Ԫ�����ڱ��еĶ�����Ԫ�أ������ʻ�ṹ��Ϣ���±���
| Ԫ�� | X | Y | Z | W | R |
| ������Ϣ | ���γ�+7�۵Ļ����� | �ճ������г����������ۻ�ʱ��������,������һ��Ĥ���� | ͨ��״�������γɶ����������ȶ���˫ԭ�ӷ��� | ��ɫ��ӦΪ��ɫ | λ�ڵ�IVA�����γɻ�������������Ԫ�� |
���û�ѧ����ش��������⣺
��1��X��Ԫ�����ڱ��е�λ����_______��
��2��Ԫ��W�����ӽṹʾ��ͼΪ_________��
��3��Ԫ��R�����������ĵ���ʽΪ_________��
��4��X���⻯���Z���⻯�ﷴӦ�γɻ�����A��A�к��еĻ�ѧ������Ϊ_________����A ����ˮ����ˮ��Һ������Ũ���ɴ�С��˳����_______________ ��
��5����Y��ij�������������ˮ���������ʵ�ˮ��Һ�����ԣ���ԭ���� ���������ӷ���ʽ��ʾ��
��6��W��������������Ҫ�Ļ�������ԭ�ϡ�д����ҵ����ȡ��������������ӷ�Ӧ����ʽ__________ ��


