��Ŀ����
�������㷺�����л��ϳɡ�ӡȾ��ҵ�ȡ���ҵ��������Ϊԭ�ϣ���Ҫ�ɷ�ΪAl��������Al2O3��Fe2O3��SiO2��CaO��MgO�ȣ��Ʊ��������Ĺ����������£�
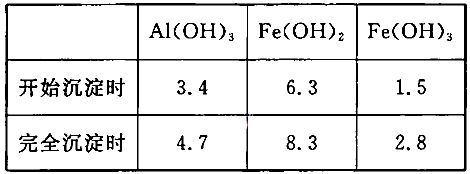
��֪��Al��OH��3�������ܽ��pH���±���
| Al��OH��3 | ��ʼ���� | ������ȫ | ������ʼ�ܽ� | �����ܽ���ȫ |
| pH | 3.3 | 5.0 | 7.8 | 12.8 |
�ش��������⣺
��1������ʱ����������Ӧ�����ӷ���ʽΪ________________________________��
��2�����������е�һ�μ��������pH��7.0��Ŀ����__________________________________________________________��
pH��7.0ʱ����Һ��c��Al3������________��ͨ�������£�Ksp[Al��OH��3]��1.3��10��33����
��3�����������ʸ��ᡢ��ķ�Ӧ�����ڼ��ܡ���pH��7.0�����ܹ����У����������ļ������ʵ���֮��n1��NaOH����n2��HNO3����n3��HNO3����________��
��4������1 t����������������[Al��NO3��3��9H2O]�����������7.5 t���������壬��������������Ԫ�ص���ʧ��Ϊ10%��������������Ԫ�ص�����������
��1��2Al��2OH����2H2O=2AlO2-��3H2��
��2��ʹAl��OH��3������ȫ��1.3��10��12 mol��L��1
��3��1��1��3
��4��60%
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ij�о�С�齫һ����������·�徭Ũ�����ϡ���ᴦ����õ�һ�����Һ,���к���Cu2+��Fe2+��Fe3+��Al3+�Ƚ�������,��������������������Էֱ���ȡCuSO4��5H2O�����AlCl3
��Һ: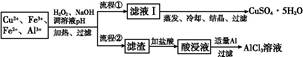
��֪:��ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ:
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
(1)����H2O2���������� ,��ʹ��ȡ��CuSO4��5H2O�����Ϊ����,pH����Ӧ��������������
(2)д��H2O2��Fe2+��Ӧ�����ӷ���ʽ: ��
(3)���̢��м�������Al����������� ��������
(4)��������ѧ�Ļ�ѧ֪ʶ,��AlCl3��Һ(������������ѧ�Լ�)�ܷ��Ƶ���ˮAlCl3��������(��ܡ����ܡ�),ԭ������ ��
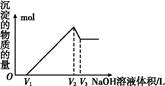
(5)ȡ���ΪV(L)�����Һ,�����еμ�a mol��L-1��NaOH��Һ,���ɳ��������ʵ��������ӵ�NaOH��Һ�����(L)��ϵ��ͼ������V1��V2��V3��ʾ��ȡ�����Һ��n(Fe3+)��n(Al3+)=�� ��
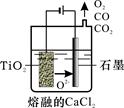
��п�����Ҫ�ɷ���̼��п��������������Fe2O3��FeO��CuO�����ʡ���ͼ������п��Ϊԭ����ȡ��������п��һ�ֹ������̣������̻����Եõ����ָ���Ʒ��������ͭ�����졣
�����±����ݣ��ش����⣺
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 6.3 | 9.7 |
| Fe3�� | 1.5 | 3.2 |
| Zn2�� | 6.2 | 8.0 |
��1�������Լ��У�________������ţ�����Ϊ�Լ�����ѡ��
A��KMnO4�� B��Cl2�� C��H2O2�� D��Ũ����
������ѡ�Լ�д����֮��Ӧ�����ӷ���ʽ��________________________________________________________________________________________________________________________________________________��
��2�������������������һЩSO42-�����ϴ��Fe��OH��3�����Լ�����ж��Ƿ�ϴ�Ӹɾ���
ϴ�ӷ�����______________________________________________________________���ж��Ƿ�ϴ�Ӹɾ��ķ�����_______________________________________��
��3�����������м��백ˮ��Ŀ���ǵ�����Һ��pH�������˵�pH��Χ��______________��������ҺpHʱ�����˰�ˮ�⣬�����Լ������������е�________��
a��Zn�� b��ZnO�� c��Zn��OH��2�� d��CuO
��4�����ⶨ����Һ���Ժ���������Fe3����Zn2������c��Fe3����Ϊ4.0��10��17 mol��L��1����c��Zn2����Ϊ______________ mol��L��1������֪Ksp[Fe��OH��3]��4.0��10��38��Ksp[Zn��OH��2]��1.2��10��17����
�Ի�ͭ��(��Ҫ�ɷ���CuFeS2,����������SiO2)Ϊԭ����ͭ�ķ�����Ϊ������ͭ��ʪ����ͭ���֡�������,ʪ����ͭ�����½�չ,��ѧ�ҷ�����һ��ϸ��������ˮ��Һ������������,���Խ���ͭ��������������:4CuFeS2+2H2SO4+17O2 4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������:
4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������:
�ش���������:
(1)���������п����������������е�����ҺpH������������(�����)��
| A��Cu�� | B��Cu2(OH)2CO3�� | C��H2SO4�� | D��Fe��E.CuO |
(3)д����Ӧ��Ļ�ѧ����ʽ:�� ��
(4)�Լ�aΪ������ ����
(5)������Һ�л���̷�����,���������ӦΪ��������(д��������)��
(6)����������,�����Լ�a��H2O����ѭ��ʹ����,����ѭ��ʹ�õ���������������(д��ѧʽ)��
 3Fe��4CO2������1 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����________ mol��
3Fe��4CO2������1 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����________ mol��
 xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3
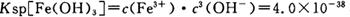 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��