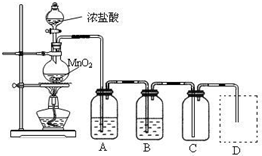
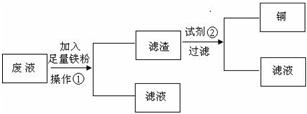
��Ŀ����
Mg��OH��2��Cu��OH��2���ܶȻ��������£�
Mg��OH��2��s��?Mg2+��aq��+2OH-��aq�� Ksp=5.6��10-12
Cu��OH��2��S��?Cu2+��aq��+2OH-��aq�� Ksp=2.2��10-20??
���¶�һ�����й�̬Mg��OH��2���ڵı�����Һ�м���ij���ʻ���Һ����ʹMg��OH��2������������ӵ��ǣ�������
Mg��OH��2��s��?Mg2+��aq��+2OH-��aq�� Ksp=5.6��10-12
Cu��OH��2��S��?Cu2+��aq��+2OH-��aq�� Ksp=2.2��10-20??
���¶�һ�����й�̬Mg��OH��2���ڵı�����Һ�м���ij���ʻ���Һ����ʹMg��OH��2������������ӵ��ǣ�������
��������������Ũ�ȶԳ����ܽ�ƽ���Ӱ���Լ���ѧʽ���ơ��ܶȻ���ij�������ת��Ϊ�ܶȻ�С�ij�����
����⣺A��CH3COONaˮ��ʹ��Һ�ʼ��ԣ�OH-��Ũ������Mg��OH��2�ij����ܽ�ƽ�������ƶ���Mg��OH��2������������ӣ���A��ȷ��
B��NH4Clˮ��ʹ��Һ�����ԣ�H+��Ũ������Mg��OH��2�ij����ܽ�ƽ�������ƶ���Mg��OH��2������������٣���B����
C��Ksp[Cu��OH��2]��Ksp[Mg��OH��2]�����¶�һ�����й�̬Mg��OH��2���ڵı�����Һ�м���CuCl2������Һ��������Mg��OH��2ת��ΪCu��OH��2��Mg��OH��2������������٣���C����
D����������ˮ��Mg��OH��2�ij����ܽ�ƽ�������ƶ���Mg��OH��2������������٣���D����
��ѡA��
B��NH4Clˮ��ʹ��Һ�����ԣ�H+��Ũ������Mg��OH��2�ij����ܽ�ƽ�������ƶ���Mg��OH��2������������٣���B����
C��Ksp[Cu��OH��2]��Ksp[Mg��OH��2]�����¶�һ�����й�̬Mg��OH��2���ڵı�����Һ�м���CuCl2������Һ��������Mg��OH��2ת��ΪCu��OH��2��Mg��OH��2������������٣���C����
D����������ˮ��Mg��OH��2�ij����ܽ�ƽ�������ƶ���Mg��OH��2������������٣���D����
��ѡA��
���������⿼�������ܵ���ʵ��ܽ�ƽ�⣬���ܽ�ƽ���Լ�ƽ��ת���ĽǶȽ��з�����ɣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
������ʵ���������������ȷ���ǣ�������
| A�������Ľ���Na��¶�ڿ����У������䰵 | B����SO2ͨ��Ʒ����Һ��Ʒ����Һ��ɫ | C����Mg��OH��2����Ͷ��FeCl3��Һ�У����������� | D��Ũ������Cu��ϼ��ȣ������а�ɫ�������� |
 ���û�ѧ��Ӧԭ���о��������⣺
���û�ѧ��Ӧԭ���о��������⣺ 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��