��Ŀ����
4�� ijͬѧ������4mol•L-l�����ᣬ��ʵ����ֻ�����ֲ�ͬŨ�ȵ������280ml��1mol•L-l�����220mL 25%���ᣨp=l.18g��mL-l������������18mol•L-l��Ũ���ᣮ
ijͬѧ������4mol•L-l�����ᣬ��ʵ����ֻ�����ֲ�ͬŨ�ȵ������280ml��1mol•L-l�����220mL 25%���ᣨp=l.18g��mL-l������������18mol•L-l��Ũ���ᣮ��1����ͬѧ���٢���������ȫ�����꣬���㲿�����ɢ۲��䣬����ѡ������ƿ�Ĺ��Ϊc������ĸ����
a.250ml�� b.500ml�� c.1000ml��
��2��������������ʵ���Ϊ0.28mol��������������ʵ���Ũ��Ϊ3mol•L-l��
��3�����ݣ�1������ѡ����ƿ�����Ҫ�۵����Ϊ170 mL��
��4�������ƹ���������Ҫ�IJ����������ձ�����Ͳ������������ͷ�ιܺ�1000mL����ƿ��
��5������ͬѧ���ý�ͷ�ιܵμ�����ˮʱ���۲�Һ��������ͼ��ʾ����������ҺŨ�Ƚ�ƫ�ߣ��ƫ�ߡ���ƫ�͡����䡱����ͬ������û��ϴ���ձ��Ͳ���������������ҺŨ�Ƚ�ƫ�ͣ���������ˮʱ���������˿̶ȣ���������ҺŨ�Ƚ�ƫ�ͣ�
���� ��1������������Һ�����ѡ����ʵ�����ƿ��
��2��������n=CV������������ʵ�����������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
��3������4mol•L-l������1000mL����������ʵ���Ϊ4mol•L-l��1L=4mol��������Һϡ������������Һ�����ʵ���������㣻
��4����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����ʵ�������
��5���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1�����������֪��280ml��1mol•L-l���ᣬ220mL 25%���ᣨp=l.18g��mL-l�����������Ϊ500mL�����㲿������������18mol•L-l��Ũ���Ჹ�䣬������Һ�������500mL������Ӧѡ��1000mL����ƿ��
��ѡ��c��
��2��������������ʵ���n=0.280L��1mol•L-l=0.28mol��������������ʵ���Ũ��Ϊ$\frac{1000��25%��1.18}{98}$=3mol/L��
�ʴ�Ϊ��0.28��3��
��3������Ҫ18mol•L-l��Ũ�������V��������Һϡ������������Һ�����ʵ��������4mol=0.28mol+0.22L��3mol/L+V��18mol/L�����V=0.17L����170mL��
�ʴ�Ϊ��170��
��4������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݣ��õ�����������Ͳ����ͷ�ιܡ����������ձ���1000mL����ƿ��
���Ի���Ҫ������Ϊ����ͷ�ιܺ�1000mL����ƿ��
�ʴ�Ϊ����ͷ�ιܺ�1000mL����ƿ��
��5������ͬѧ���ý�ͷ�ιܵμ�����ˮʱ���۲�Һ��������ͼ��ʾ�����ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
��û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
��������ˮʱ���������˿̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ͣ�ƫ�ͣ�
���� ���⿼��������һ�����ʵ���Ũ�ȵ����Ƽ��㣬������ѵ���������ƿ����ѡ����ȷ����ԭ���ǽ���ؼ�����Ŀ�ѶȲ���

 �������ϵ�д�
�������ϵ�д�| A�� | ��������ɹ㷺����ֽ����ë��˿����ñ��ȵ�Ư�� | |
| B�� | ������ˮ��ԭ���͡�84������Һ������ԭ������ͬ | |
| C�� | ʳ��ȥ��ˮ����NH4Cl��Һ��ȥ������ | |
| D�� | CO2��NO2����ʹ��ˮ��pH��5.6������������γ� |
| A�� | Cu2+��H+��Cl- | B�� | Na+��CO32-��Cl- | C�� | Na+��H+��Cl- | D�� | Na+��OH-��CO32- |
| A�� | �������ձ� | B�� | �Թܡ�ȼ�ճ� | C�� | ������ƽ����ƿ | D�� | ������Բ����ƿ |
| A�� | ��������BaCl2��Һ����� | B�� | ��������NaOH��Һ����� | ||
| C�� | �������NaOH��Һ����� | D�� | �������Ba��OH��2��Һ����� |
| A�� | ij��Һ�м��� Ba��NO3��2��Һ���ٵμ�ϡ���ᣬ���ְ�ɫ�����Ҳ��ܽ⣬��ԭ��Һ�п��ܺ��� SO${\;}_{3}^{2-}$ | |
| B�� | ij������ʹʪ�����ɫʯ����ֽ��죬�������һ�������������� | |
| C�� | �Ҵ��л������ᣬ���������ƺ����ɵõ��������Ҵ� | |
| D�� | �ڷ�Һ©���м�����ˮ��Ȼ���ټ������������Ȼ�̼�����ã������ȡ�����Ȼ�̼�� |
| A�� | ����Ҫ���Ⱦ��ܷ����ķ�Ӧһ���Ƿ��ȷ�Ӧ | |
| B�� | ���������Һ�ĵ�������һ����ǿ�������Һ���� | |
| C�� | SO2����ˮ����ˮ��Һ�ܵ��磬��SO2�ǵ���� | |
| D�� | �����¶ȣ��������Ӱٷ�����ʹ��Ч��ײ���࣬��ѧ��Ӧ���ʼӿ� |
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
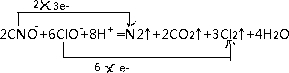 ��
��