��Ŀ����
1����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25mol/L���ᵹ��С�ձ��У������¶ȣ�����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȣ���ش���1��NaOH��Һ�Թ�����ԭ��ȷ�����ᱻ��ȫ�кͣ�
��2������NaOH��Һ����ȷ������B ������ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�������������¶ȼ��ϵĻ��β������������ؽ�����
��4������Һ���ܶȾ�Ϊ1g•cm-3���кͺ���Һ�ı���c=4.18J•��g•�棩-1�������ʵ����������к���Ϊ56.85kJ/mol��д���÷�Ӧ���Ȼ�ѧ����ʽH2SO4��aq��+2NaOH��aq��=Na2SO4��aq��+2H2O��H=-113.7kJ/mol��
| �¶�ʵ����� | ��ʼ�¶�t2 /�� | ��ֹ�¶� t2 /�� | �¶Ȳ�ƽ��ֵ ��t2- t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 25.0 | 25.2 | 25.1 | 28.5 | 3.4 |
| 2 | 24.9 | 25.1 | 25.0 | 28.3 | 3.3 |
| 3 | 25.6 | 25.4 | 25.5 | 29.0 | 3.5 |
���� ��1��Ϊ��ȷ�����������ᷴӦ��ȫ������NaOH�Թ�����
��2����NaOH��Һ����С�ձ��У����ּܷ��ε��룬����ᵼ������ɢʧ��Ӱ��ⶨ�����
��3��������������ƻ��ʱ���������¶ȼ��ϵĻ��β������������ؽ�����ʹ������NaOH��Һ��Ͼ��ȣ�
��4���ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ������Ȼ�ѧ����ʽ����д��������д��
��� �⣺��1��ʵ���У�����NaOH�Թ�����ԭ����ȷ�����ᷴӦ��ȫ���ʴ�Ϊ��ȷ�����ᱻ��ȫ�кͣ�
��2����������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ�������ѡ��B��
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ������ʴ�Ϊ���������¶ȼ��ϵĻ��β������������ؽ�����
��4��50mL 0.25mol/L������50mL 0.55mol/L NaOH��Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.25mol/L��2=0.025mol����Һ������Ϊ100ml��1g/ml=100g���¶ȱ仯��ֵΪ����ʵ���ƽ��ֵ3.4�棬������0.025molˮ�ų�������ΪQ=m•c•��T=100g��4.18J/��g•�棩��3.4��=1421.2J����1.421kJ��
����ʵ���õ��к��ȡ�H=-$\frac{1.421kJ}{0.025mol}$=-56.85kJ/mol���÷�Ӧ���Ȼ�ѧ����ʽH2SO4��aq��+2NaOH��aq��=Na2SO4��aq��+2H2O��H=-113.7kJ/mol��
�ʴ�Ϊ��56.85kJ/mol��H2SO4��aq��+2NaOH��aq��=Na2SO4��aq��+2H2O��H=-113.7kJ/mol��
���� ���⿼���к��ȵIJⶨ����㣬��Ŀ�Ѷ��еȣ�ע�������к��ȵĸ����ǽ���Ĺؼ���

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�| A�� | ��CH3��2CHCH2CH2CH3 | B�� | ��CH3��2CHCH3 | C�� | ��CH3��2CH-CH��CH3��2 | D�� | ��CH3��3CCH2CH3 |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� |

��2���ؿ��к������Ľ���Ԫ����Al
��3���õ���ʽ��ʾ������γɻ�����Ĺ���
 ��
����4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH�������Ե�����������Al��OH��3��
��5��д�������������Ʒ�Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
д���ݵ���������������������Һ��Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
| A�� | ��Ba��OH��2��Һ�еμ�ϡ���Ba2++OH-+H++SO42-�TBaSO4��+H2O | |
| B�� | ��Na��Ͷ��ˮ�У��������壺Na+2H2O�TNa++2OH-+H2�� | |
| C�� | 0.1 mol•L-1������������Һ��pHԼΪ3��HSO3-+H2O�TSO32-+H3O+ | |
| D�� | ��Al2��SO4��3��Һ�м��������NH3•H2O��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ |
| A�� | 46g�Ҵ��к��й��ۼ�����ĿΪ7NA | |
| B�� | 50ml 2mol•L-1NaClO��Һ��ClO-��ĿΪ0.1NA | |
| C�� | ��״���£�5.6gFe��������Ũ�����ַ�Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| D�� | ���³�ѹ�£�4.4g��CO2��N2O��ɵĻ����������ԭ������Ϊ0.3NA |
| A�� | ��ȡ10.6 g��ˮ̼���ƣ�����100 mL����ƿ�У���ˮ�ܽ⡢���� | |
| B�� | ���ݺ�����ƿ����������ת��ҡ�� | |
| C�� | ת��Na2CO3��Һʱ��δ�ò�����������ֱ�ӵ�������ƿ�� | |
| D�� | ��ȡ10.6 g��ˮ̼���ƣ�����100 mL����ˮ�����衢�ܽ� |
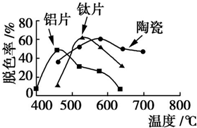 �ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
�ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��ͬ���壬���ۺ����¶�һ������Ƭ����Ĺ��������� | |
| B�� | ���ۺ������壬�������������¶ȵ����߶����� | |
| C�� | Լ��520��ʱ����Ƭ����Ĺ��������� | |
| D�� | ��ͬ���壬TiO2��Ĥ�Ĺ��������ͬ |
 �����ٴ���һ�ֽ�֬��Ѫ˨ҩ�����һ���ϳ�·����ͼ��
�����ٴ���һ�ֽ�֬��Ѫ˨ҩ�����һ���ϳ�·����ͼ��

 ��
��