��Ŀ����
15����ͼΪԪ�����ڱ���һ���֣������Ԫ�آ�-����ͼ�е�λ�ã�����ػ�ѧ����ش��������⣺��ע�⣺����Żش��֣�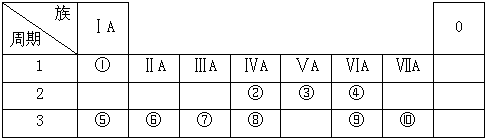
��1����Ԫ���⻯��ĵ���ʽ��
 ��
����2���ڡ��ۡ���Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��ΪMg��C��N��
��3���ۡ��ܡ���Ԫ�ص���̬�⻯���ȶ�����ǿ������˳����H2O��NH3��SiH4��
��4���ڡ��ܡ���Ԫ�صķǽ�������ǿ������˳����O��C��Si��
��5���ݡ�������Ԫ�ص�����������Ӧ��ˮ������ˮ��Һ���ܷ������ӷ�Ӧ����÷�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Mg������Al������Si������S������Cl��
��1����Ԫ���⻯��Ϊˮ��
��2�����Ӳ�Խ��뾶Խ���Ӳ���ͬʱ�˵����Խ��뾶ԽС��
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��4��ͬ����ԭ��������ķǽ�����ǿ��ͬ����ԭ������С�ķǽ�����ǿ��
��5���ݡ�������Ԫ�ص�����������Ӧ��ˮ������ˮ��Һ�У���Ӧ����ƫ�����ƺ�ˮ��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Mg����Al������Si������S������Cl��
��1����Ԫ���⻯��Ϊˮ�������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2�����Ӳ�Խ��뾶Խ���Ӳ���ͬʱ�˵����Խ��뾶ԽС����ԭ�Ӱ뾶ΪMg��C��N���ʴ�Ϊ��Mg��C��N��
��3���ǽ�����ΪO��N��Si����̬�⻯����ȶ���ΪH2O��NH3��SiH4���ʴ�Ϊ��H2O��NH3��SiH4��
��4��ͬ����ԭ��������ķǽ�����ǿ��ͬ����ԭ������С�ķǽ�����ǿ����ڡ��ܡ���Ԫ�صķǽ�������ǿ������˳����O��C��Si���ʴ�Ϊ��O��C��Si��
��5���ݡ�������Ԫ�ص�����������Ӧ��ˮ������ˮ��Һ�У���Ӧ����ƫ�����ƺ�ˮ�����ӷ�ӦΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʼ�Ԫ�ػ�����֪ʶ���ۺ�Ӧ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�| ѡ�� | ʵ����� | ���� | ���� |
| A | ����ɫ��Һ�еμ���ˮ��CCl4������ | �²���Һ�� ��ɫ | ԭ��Һ�к���I- |
| B | �������ۼ���ϡ�����г�ַ�Ӧ���ټ���KSCN��Һ | ��Һ�ʺ�ɫ | ϡ���Ὣ�� ����ΪFe2+ |
| C | �������Һ�еμ�ϡ���ᣬ���ȣ�ȡˮ��Һ�������μ�������Һ����ˮԡ���� | û���������� | ����û�з���ˮ�� |
| D | �ò�����պȡŨ����ε���ɫʯ����ֽ�� | ��ֽ��� | Ũ���������ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Al2��SO4��3��NaHCO3 | B�� | Na2SO4��NH4HCO3 | C�� | MgSO4��Na2CO3 | D�� | NaHCO3��NaHSO4 |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� | �� |
��2���ߵĻ�̬ԭ�ӵ����Ų�ͼ
 ��
����3����ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽF2+2H2O=4HF+O2��
��4����ʾ����ߵĻ��������ɼ��ԣ�����ԡ������Ǽ��ԡ������γɵķǼ���
������ԡ������Ǽ��ԡ������ӣ�
��5���ڵĵ��ʵĵȵ����з�����CO��������C22-������������γɵĻ�������ˮ��Ӧ�Ļ�ѧ����ʽCaC2+2H2O��Ca��OH��2+C2H2����
��6���ڵ��⻯�K������ˮ������һ��ԭ��������ˮ֮����γ������д������ˮ��Һ����������ı���ʽN-H��N��O-H��O��N-H��O��O-H��N��
| A�� | ���������ӵ�Ũ��Ϊ0.2mol•L-1 | |
| B�� | ��Һ�е�OH-Ũ�ȴ���H+Ũ�ȣ�HCO3-��Ũ��С��CO32-��Ũ�� | |
| C�� | ��Һ��Na+��Ũ�ȵ���CO32-Ũ�ȵĶ��� | |
| D�� | ��Һ��H2CO3���ӵ�Ũ��Ϊ0 |
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | ⑪ | �� | �� | ||
| �� | �� | �� |
 ��
����2���ؿ��к������Ľ���Ԫ����Al��д��Ԫ�آ��⻯��Ļ�ѧʽCH4��
��3����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH�������Ե�����������Al��OH��3��
��4��д���ۣ��ܣ��ݶ�Ӧ������������ǿ��������Al3+��Mg2+��Na+���ڡ��ߡ����Ӧ�⻯��������ǿ��������HBr��HCl��HF��
��5��д��⑪���������Ʒ�Ӧ�Ļ�ѧ����ʽ��Si+2NaOH+H2O=Na2SiO3+2H2����
 ��ͼ��ʾ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ���Wԭ�����������������ڲ��������$\frac{7}{10}$��������˵���в���ȷ���ǣ�������
��ͼ��ʾ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ���Wԭ�����������������ڲ��������$\frac{7}{10}$��������˵���в���ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�ɴ�С����˳��Z��X��Y | |
| B�� | YԪ�ص�����ͬ���������ڳ����¶������� | |
| C�� | ����������Ӧˮ���������W��Z | |
| D�� | �����Ӱ뾶�ɴ�С����˳��X��Y��Z��W |
| A�� | ���³�ѹ��46g N02��N204��������к��е�ԭ����Ϊ3NA | |
| B�� | ��1mol CH3COONa������CH3COOH�γɵ�������Һ�У�CH3COO-��ĿΪNA | |
| C�� | ���³�ѹ�£�1mol�л���C2H60�к��м��Լ�����Ŀһ��Ϊ7NA | |
| D�� | һ�������£�2mo1 SO2��1mol 02�ڻ���ܱ������г�ַ�Ӧ�������еķ���������2NA |
| A�� | ������ ������Ϊ2-��-5-�һ����� ������Ϊ2-��-5-�һ����� | |
| B�� | ʯ�ʹ��ѻ�����ҪĿ����������͵������͵IJ��� | |
| C�� | �ڵ�������Һ�м�����������Һ�������֣�����ʹ�����ʱ��ԣ�ʧȥ�������� | |
| D�� | CH3COOCH2CH3��CH3CH2CH2COOH��Ϊͬ���칹�壬��ȩ�ͱ���ȩ��Ϊͬϵ�� |