��Ŀ����
��15�֣�����������ι�ҵ����Ҫ��Ʒ֮һ����ͨ����Ӧ��NaC1O3+KC1 KC1O3��+NaC1��ȡ��
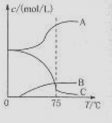
��1��ʵ������ȡ�����ƿ�ͨ����Ӧ��3C12+6NaOH 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
��2����ҵ����ȡ�����Ʋ������ȵ�ʯ����ͨ��������Ȼ
��ᾧ��ȥ�Ȼ��ƺ��ټ���һ�����Σ����ʵ�������
��
��3��������ŷ���������������ö���������ˮ������
��Ĥ��ⷨ��ã������������漰����Ҫ�Ļ�ѧ��Ӧʽ���£�
�ܷ�Ӧʽ��NaC1+3H2O NaC1O3+3H2��
NaC1O3+3H2��
������2C1����2e�� C12��������2H2O+2e�� H2��+2OH��
Һ�෴Ӧ��C12+H2O HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O H++C1O��
H++C1O��
2HC1O+CO�� C1O3��+2C1��+2H+
����ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����μ���Ļ�ѧ�Լ�
�� �� �� �����ˣ���Һ���ټ���������ϡ���ᣬ��һ��������ˮ�پ����ӽ���������Ĥ�����õ�����������ˮ��
�ڵ��ʱ��������ʳ��ˮ�м���Na2Cr2O7����Ŀ���Ƿ�ֹ �������ӷ��ţ����������������Ϸŵ硣
��4����NaC1O3��KC1�Ļ����Һ��NaC1O3��KC1�����������ֱ�Ϊ0.290��0.203��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�KC1O3�����ʵ���������
Ϊ ����������ƣ������
��5����Ʒ��C1O3���ĺ������õζ������вⶨ��ʵ�鲽��
���£�
����1��ȷ��ȡ��Ʒag��Լ2.20g�������ܽ⡢���ݵȲ���ȷ����1000mL��Һ��
����2������������ƿ��ȡ��10.00mL����ƿ�У�ȷ����25mL1000mol/L(NH4)2Fe��SO4��2����Һ��������������75mL�����������ɵĻ��ᣬ����10min��
����3��������ƿ�м���100mL����ˮ��ij��������ԭ��Ӧָʾ������0.200mol/LK2Cr2O7����Һ�ζ����յ㡣
����4�� ��
����5�����ݴ�������㡣
�ٲ���2������10min��Ŀ���� ��
�ڲ���3��K2Cr2O2����ҺӦʢ���� �У����������ƣ���
��Ϊ��ȷ����Ʒ��C1O3������������������4�IJ��������� ��
��1��ʵ������ȡ�����ƿ�ͨ����Ӧ��3C12+6NaOH
 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
��2����ҵ����ȡ�����Ʋ������ȵ�ʯ����ͨ��������Ȼ
��ᾧ��ȥ�Ȼ��ƺ��ټ���һ�����Σ����ʵ�������
��
��3��������ŷ���������������ö���������ˮ������
��Ĥ��ⷨ��ã������������漰����Ҫ�Ļ�ѧ��Ӧʽ���£�
�ܷ�Ӧʽ��NaC1+3H2O
 NaC1O3+3H2��
NaC1O3+3H2��������2C1����2e��
Һ�෴Ӧ��C12+H2O
 HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O H++C1O��
H++C1O��2HC1O+CO��
����ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����μ���Ļ�ѧ�Լ�

�� �� �� �����ˣ���Һ���ټ���������ϡ���ᣬ��һ��������ˮ�پ����ӽ���������Ĥ�����õ�����������ˮ��
�ڵ��ʱ��������ʳ��ˮ�м���Na2Cr2O7����Ŀ���Ƿ�ֹ �������ӷ��ţ����������������Ϸŵ硣
��4����NaC1O3��KC1�Ļ����Һ��NaC1O3��KC1�����������ֱ�Ϊ0.290��0.203��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�KC1O3�����ʵ���������
Ϊ ����������ƣ������
��5����Ʒ��C1O3���ĺ������õζ������вⶨ��ʵ�鲽��
���£�
����1��ȷ��ȡ��Ʒag��Լ2.20g�������ܽ⡢���ݵȲ���ȷ����1000mL��Һ��
����2������������ƿ��ȡ��10.00mL����ƿ�У�ȷ����25mL1000mol/L(NH4)2Fe��SO4��2����Һ��������������75mL�����������ɵĻ��ᣬ����10min��
����3��������ƿ�м���100mL����ˮ��ij��������ԭ��Ӧָʾ������0.200mol/LK2Cr2O7����Һ�ζ����յ㡣
����4�� ��
����5�����ݴ�������㡣
�ٲ���2������10min��Ŀ���� ��
�ڲ���3��K2Cr2O2����ҺӦʢ���� �У����������ƣ���
��Ϊ��ȷ����Ʒ��C1O3������������������4�IJ��������� ��

��1���ڡ�5���NaOH��Һ��ͨ������C12ʱ������Ӧ�ķ���ʽΪ��CL2 + 2NaOH ===== NaCL + NaCLO + H2O����ʱCL2������������Ҫ��Ҫ��NaCLO������Һ���ȣ����¶ȵIJ��ϳ��ߣ�����Ӧ����ʽΪ��3C12+6NaOH 5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3��������
5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3��������
��2���ȵ�ʯ����ͨ����������Ӧ�ķ���ʽΪ��2CL2 + 2Ca(OH)2===== CaCL2 + Ca(CLO)2 + 2H2O����ȥCaCL2����Ҫ�ɷ�ΪCa(CLO)2��Ҫ�������εõ�NaCLO3��������ѭ���ֽⷴӦ�������������������ο�����Na2SO4��Na2CO3��
��3������ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����Լ�Ϊ��Na2CO3��ȥCa2+��NaOH��ȥMg2+��BaCL2��ȥSO42��������������Լ���ѭ��ԭ���ǣ�Na2CO3һ������BaCL2֮��HCLһ���������
���ʱ��������ʳ��ˮ�м���Na2Cr2O7����Ŀ���Ƿ�ֹCLO-�ڵ��������������ŵ磻
��4�����ݸ����ʵ��ܽ������ͼ������֪��Ҫ��NaC1O3��KC1�Ļ����Һ�еõ�KC1O3�����õĴ�ʩΪ������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�
��5��K2Cr2O2����Һ����ǿ�����ԣ����������ʽ�ζ����У���Ϊǿ���������ʻḯʴ��ʽ�ζ��ܵ��ܣ�����10min��Ŀ����ʹ��Һ�е�CLO-��ȫ��Fe2+��ԭΪCL-��Ϊ��ʹ��ʵ������������������һ�����ظ���������2�Ͳ���3�������Ρ�
 5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3��������
5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3����������2���ȵ�ʯ����ͨ����������Ӧ�ķ���ʽΪ��2CL2 + 2Ca(OH)2===== CaCL2 + Ca(CLO)2 + 2H2O����ȥCaCL2����Ҫ�ɷ�ΪCa(CLO)2��Ҫ�������εõ�NaCLO3��������ѭ���ֽⷴӦ�������������������ο�����Na2SO4��Na2CO3��
��3������ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����Լ�Ϊ��Na2CO3��ȥCa2+��NaOH��ȥMg2+��BaCL2��ȥSO42��������������Լ���ѭ��ԭ���ǣ�Na2CO3һ������BaCL2֮��HCLһ���������
���ʱ��������ʳ��ˮ�м���Na2Cr2O7����Ŀ���Ƿ�ֹCLO-�ڵ��������������ŵ磻
��4�����ݸ����ʵ��ܽ������ͼ������֪��Ҫ��NaC1O3��KC1�Ļ����Һ�еõ�KC1O3�����õĴ�ʩΪ������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�
��5��K2Cr2O2����Һ����ǿ�����ԣ����������ʽ�ζ����У���Ϊǿ���������ʻḯʴ��ʽ�ζ��ܵ��ܣ�����10min��Ŀ����ʹ��Һ�е�CLO-��ȫ��Fe2+��ԭΪCL-��Ϊ��ʹ��ʵ������������������һ�����ظ���������2�Ͳ���3�������Ρ�

��ϰ��ϵ�д�
�����Ŀ
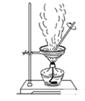
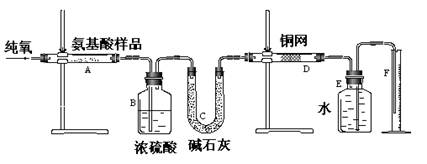
 ��죬�ּ�����ʹ�����ǵ�ľ����ȼ������0.28L������£���
��죬�ּ�����ʹ�����ǵ�ľ����ȼ������0.28L������£���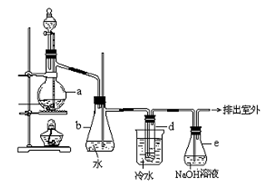
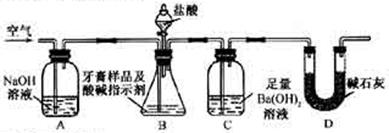
 ��ijƷ��������Ħ�����ɷּ��京����������̽����
��ijƷ��������Ħ�����ɷּ��京����������̽���� __________________________________________��
__________________________________________��
 ___��
___��