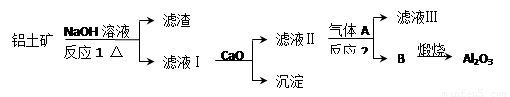��Ŀ����
Na3AlF6������ˮ��������θ���Ժʹ�ɱ��ɱ������´��������֬����ĥ���������㷺Ӧ��������ұ������ҵ������Na3AlF6����������
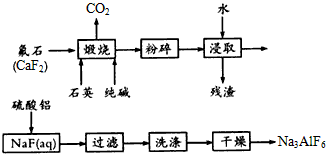
�Խ���������̻ش���������
(1)����ʱ�ܷ�Ӧ�Ļ�ѧ����ʽΪ____________________��
(2)���պ�Ĺ����ڽ�ȡǰ���з����Ŀ����____________��Ϊ�ﵽ��ͬĿ�ģ����½�ȡʱ���ɲ�ȡ�Ĵ�ʩ��____________��
(3)97.5 kg��CaF2 80%��ʯ�����ʲ�����Ԫ�أ������Ͽ�����Na3AlF6______kg�������������е�ÿһ���������ʾ���ȫת������
(4)�ڰ���ѧ����������������Һǰ��������________��NaF(aq)��pH�µ���5���ң�������ܲ����ĸ�����Ϊ________��
(5)������ұ���У�Na3AlF6��һ�����ۼ�����ʹAl2O3��1000���������ڣ�������ǿ��ϵ�ĵ����ԡ������й�Na3AlF6���ʵ���������ȷ����________��
a��Na3AlF6��ˮ�в����ڳ����ܽ�ƽ��
b��Na3AlF6�����ӻ�����
c��Na3AlF6���ȶ���ǿ
d��Na3AlF6��һ�ַǵ����
(1)����ʱ�ܷ�Ӧ�Ļ�ѧ����ʽΪ____________________��
(2)���պ�Ĺ����ڽ�ȡǰ���з����Ŀ����____________��Ϊ�ﵽ��ͬĿ�ģ����½�ȡʱ���ɲ�ȡ�Ĵ�ʩ��____________��
(3)97.5 kg��CaF2 80%��ʯ�����ʲ�����Ԫ�أ������Ͽ�����Na3AlF6______kg�������������е�ÿһ���������ʾ���ȫת������
(4)�ڰ���ѧ����������������Һǰ��������________��NaF(aq)��pH�µ���5���ң�������ܲ����ĸ�����Ϊ________��
(5)������ұ���У�Na3AlF6��һ�����ۼ�����ʹAl2O3��1000���������ڣ�������ǿ��ϵ�ĵ����ԡ������й�Na3AlF6���ʵ���������ȷ����________��
a��Na3AlF6��ˮ�в����ڳ����ܽ�ƽ��
b��Na3AlF6�����ӻ�����
c��Na3AlF6���ȶ���ǿ
d��Na3AlF6��һ�ַǵ����
(1) CaF2+SiO2+Na2CO3=CaSiO3+2NaF+CO2��
(2)���ٿ�������ܽ⣻��ֽ���
(3)70
(4)���Al(OH)3
(5)ad
(2)���ٿ�������ܽ⣻��ֽ���
(3)70
(4)���Al(OH)3
(5)ad

��ϰ��ϵ�д�
�����Ŀ
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ��
�Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�
 |
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ �� ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����֪AlCl3���۵��Al2O3���۵�͵ö࣬��ҵ�����е��Al2O3��������ұ���������ǵ�����ڵ�AlCl3��ԭ���ǣ� ��