��Ŀ����
����Ŀ��I��������һ�������Դ������Դ�����ǣ�
;��1����̫���ֽܷ�ˮ�������� ;��2���þ۽�̫���ܷ�Ӧ���������·�Ӧ��CH4(g)��H2O(g)===CO(g)��3H2(g) ��H����206 kJ��mol��1
��֪����25�桢101kPaʱ 2CO(g)+ O2 (g) ===2CO2(g) ��H����566.0 kJ��mol��1
��1����25�桢101kPaʱ��1��H2��ȫȼ��������̬ˮʱ����120.9kJ��������ȫ�÷�Ӧ���Ȼ�ѧ����ʽ��2H2��g��+O2��g��===2H2O��g����H =_________
��2����֪�����γ�1mol��ѧ����Ҫ��ͬ��������;��2�У���1mol H��H����������ckJ��1molH��O����bkJ��1mol C��H����akJ�����1mol CO(g)��ѧ����Ҫ������_______kJ ���ú���ĸ��ʽ�ӱ�ʾ��
��3��CH4ȼ������CO2����̬H2O���Ȼ�ѧ����ʽΪ__________
II������������ȼ�ϵ��Ϊ��Դ���е���ʵ��װ����ͼ��ʾ��
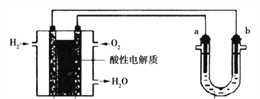
��4��ȼ�ϵ�ع���ʱ��������ӦΪ��______________��
��5��U����װCuSO4��Һʱ��a��b��������ʯī������ͬ������a���������������������ĵ�H2������ǣ�________��
��6��U����װ����NaClˮ��Һʱ������ܷ�Ӧ�����ӷ���ʽ��______________��
��7���ö��Ե缫���M��NO3��x��ˮ��Һ��������������m��ʱ����������ͬʱ����nL��������״�������Ӷ���֪M�����ԭ��������_________�����ú���ĸ��ʽ�ӱ�ʾ��
���𰸡� -483.6 kJ��mol��1 ��4a+2b-3c-206�� CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����802.4 kJ��mol��1 O2��4H����4e��===2H2O�� 1:2 2Cl -��2H2O![]() 2OH -��H2����Cl2��
2OH -��H2����Cl2�� ![]()
��������(1) ��25����101kPaʱ��1��H2��ȫȼ��������̬ˮʱ����120.9kJ��������2mol����Ϊ4g����ȫȼ��������̬ˮʱ����120.9kJ��4=483.6 kJ��������Ӧ���Ȼ�ѧ����ʽ��2H2(g)+O2(g)===2H2O(g)��H =-483.6 kJ��mol��1���ʴ�Ϊ��-483.6 kJ��mol��1��
(2)���1mol CO(g)��ѧ����Ҫ������x kJ��C H4(g)��H2O(g)===CO(g)��3H2(g) ��H����206 kJ��mol��1=��Ӧ��ļ���֮��-������ļ���֮��=4a+2b-3c-x�����x=(4a+2b-3c-206)kJ���ʴ�Ϊ��4a+2b-3c-206��
(3)��CH4(g)��H2O(g)===CO(g)��3H2(g) ��H����206 kJ��mol��1����2CO(g)+ O2 (g) ===2CO2(g) ��H����566.0 kJ��mol��1 ����2H2(g)+O2(g)===2H2O(g)��H =-483.6 kJ��mol��1�����ݸ�˹���ɣ�����+![]() ����+
����+![]() ���۵ã�CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H��(��206 kJ��mol��1)+
���۵ã�CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H��(��206 kJ��mol��1)+![]() ��(��566.0 kJ��mol��1)+
��(��566.0 kJ��mol��1)+![]() ��(-483.6 kJ��mol��1)=-802.4 kJ��mol��1���ʴ�Ϊ��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����802.4 kJ��mol��1��
��(-483.6 kJ��mol��1)=-802.4 kJ��mol��1���ʴ�Ϊ��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����802.4 kJ��mol��1��
(4)���ȼ�ϵ���������Ե�����й����������������õ�������ˮ������������ӦΪ��O2+4e-+4H+=2H2O���ʴ�Ϊ��O2+4e-+4H+=2H2O��
(5)U����װCuSO4��Һʱ��a��b��������ʯī��a��Ϊ��������Һ�е�ˮʧȥ��������������2H2O��4e-=O2��+4H+�����ݵ�ʧ�����غ㣬a������1mol����ת��4mol���ӣ����������ĵ�H22mol���������ͬ������a���������������������ĵ�H2�������1:2���ʴ�Ϊ��1:2��
(6)U����װ����NaClˮ��Һʱ����ⱥ��NaClˮ��Һ���ܷ�Ӧ�����ӷ���ʽΪ2Cl -��2H2O![]() 2OH -��H2����Cl2�����ʴ�Ϊ��2Cl -��2H2O
2OH -��H2����Cl2�����ʴ�Ϊ��2Cl -��2H2O![]() 2OH -��H2����Cl2����
2OH -��H2����Cl2����
(7)��M��ԭ����ΪR�����ʱ����ط�Ӧ����ʽΪ��
4M(NO3)x+2xH2O![]() 4M + xO2��+4xHNO3
4M + xO2��+4xHNO3
4Rg 22.4xL
mg nL
����R=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

����Ŀ����ͬ�¶��£������Ϊ0.25 L�������ܱ������з�����Ӧ��X2(g)��3Y2(g) ![]() 2XY3(g)����H����92.6 kJ��mol��1ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��
2XY3(g)����H����92.6 kJ��mol��1ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��
��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | |||
���� | X2 | Y2 | XY3 | |
�����ٺ��º��� | 1 | 3 | 0 | ���� 23.15 kJ |
�����ں��º�ѹ | 1 | 3 | 0 | Q(Q��0) |
����������ȷ���ǣ� ��
A. �����١����з�Ӧ��X2��ת������ͬ
B. ��ƽ��ʱ������������XY3�����ʵ���Ũ�Ⱦ�Ϊ2 mol��L��1
C. �����١��ڴﵽƽ��ʱ����ͬ
D. �����ڣ� Q����23��15 kJ
����Ŀ��NaOH�����ᶼ����ѧ��ѧ�������Լ�
��һ��ijͬѧ��0.200 0 mol��L��1������ζ������ռ���ҺŨ��
��1����5.0g�ռ���Ʒ( ���ʲ����ᷴӦ)���250 mL����Һ��ȡ10.00 mL����Һ����________ʽ�ζ�����ȡ����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________ mL��

��2�����±����ݣ� NaOH�İٷֺ�����________��
�ζ����� | ����NaOH��Һ���(mL) | ��������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��3���ñ�����ζ�ijŨ�ȵ�NaOH��Һ�����в�������ʹ�ⶨ���ƫ�ߵ�����_____��
A��������ˮϴ��ƿ��ֱ��ȡ10.00 mL����Һע����ƿ��
B����ʽ�ζ�����װ��Һǰδ�ñ�������Һ��ϴ2��3��
C����ʼʵ��ʱ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ�������������ʧ
D������ʽ�ζ��ܣ��ζ�ǰ��ȷ�������ζ����Ӷ���
������50 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ�ⶨ�����кͷ�Ӧ�ķ�Ӧ�ȡ��ش��������⣺

��4����ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ�����Ʒ��__________�������0.0275 mol NaOH��������������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��________( �ƫ����ƫС������Ӱ�족)��
��5����֪�����NaOHϡ��Һ�����кͷ�Ӧ����0.1 mol H2Oʱ���ų�5.73 kJ�����������ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽΪ��_______________��
����������ʱ����ʢ��0.1 mol��L��1Mg2+��Һ���Թ��еμ�������NaOH��Һ����pHΪ11.0ʱ����֪Ksp Mg(OH)2=5.61��1012��
��6����ʱ�ϲ���Һ��c��Mg2+��=________ mol��L��1
����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)����ϵ��n(NO2)��ʱ��ı仯�����
2NO2(g)����ϵ��n(NO2)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO2)(mol) | 0.00 | 0.010 | 0.012 | 0.013 | 0.013 | 0.013 |
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ��K=____________����֪��K��350����300��������÷�Ӧ��________��������ţ��ȷ�Ӧ��
��2����ͼ�б�ʾNO�ı仯��������_________������ĸ����O2��ʾ��0~2 s�ڸ÷�Ӧ��ƽ������v=_________��
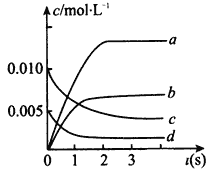
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����______________������ĸ��
A��c(NO2)=2c(O2) B��������ѹǿ���ֲ���
C��v�� (NO)=2v��(O2) D���������ܶȱ��ֲ���