��Ŀ����
��������ʮ�����ʣ���H2 ���� ��CuO ��CO2 ��H2SO4 ��Ba��OH��2 �ߺ��ɫ����������Һ�� �ఱˮ ��ϡ�����Al2��SO4��3
��1�������ʵķ������д����Ŀհ״���
| ����� | ������ | ����� | |||
| ���ڸ�������� | �� | ��� | �� |
��3������ˮ�еĵ��뷽��ʽΪ______��17.1g������ˮ���250mL��Һ��SO42-��������Ϊ______��SO42-�����ʵ���Ũ��Ϊ______��
��4�������Ģ�ͨ�����Һ�з�Ӧ�Ļ�ѧ����ʽΪ______��
�⣺��1���������ʵ�Ԫ����ɣ����Խ����ʷ�Ϊ������ͻ����������У��������Ԫ�ص�������Խ����ʷ�Ϊ���ʺͻ����ֻ��һ��Ԫ����ɵĴ������ǵ��ʣ�������������ݻ���������ʣ����Խ��������Ϊ�ᡢ��Ρ������������ͭ����������̼������������ݻ�����ɢ����ֱ���Ĵ�С�����Խ�������Ϊ��Һ���簱ˮ��ϡ���ᣩ��Һ�ͽ��壨����ɫ����������Һ�壩���֣���������ˮ��Һ��������̬���Ƿ磬���Խ��������Ϊ����ʺͷǵ���ʣ���CuO��H2SO4��Ba��OH��2��Al2��SO4��3���ڵ���ʣ��ʴ�Ϊ��
��2����ͼ�֮����Է����кͷ�Ӧ�����κ�ˮ����������������֮�ķ�ӦΪ��Ba��OH��2+2HNO3=Ba��NO3��2+2H2O���ʴ�Ϊ��Ba��OH��2+2HNO3=Ba��NO3��2+2H2O��
��3����������ǿ����ʣ�����ȫ���룬���뷽��ʽΪ��Al2��SO4��3=2Al3++3SO42-��17.1gAl2��SO4��3����ˮ���250mL��Һ��SO42-��������Ϊ =9.03��1022��SO42-�����ʵ���Ũ��c=
=9.03��1022��SO42-�����ʵ���Ũ��c=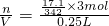 =0.6mol/L���ʴ�Ϊ��9.03��1022��0.6mol/L��
=0.6mol/L���ʴ�Ϊ��9.03��1022��0.6mol/L��
��4�������Ķ�����̼���Ժ�ǿ������������Ӧ����̼�ᱵ��ˮ����Ba��OH��2+CO2=BaCO3��+H2O��
�ʴ�Ϊ��Ba��OH��2+CO2=BaCO3��+H2O��
��������1���������ʵķ�����Լ������������ж��������������
��2����ͼ�֮����Է����кͷ�Ӧ�����κ�ˮ��
��3�����ݵ��뷽��ʽ����д����֪ʶ���ش𣬸��ݹ�ʽN=nNA= NA�Լ�c=
NA�Լ�c= ������ش�
������ش�
��4�������Ķ�����̼���Ժ�ǿ�Ӧ����̼���κ�ˮ��
������������һ���������ʵķ��ࡢ���ʵ����ļ����Լ����ʵ����ʷ���֪ʶ���ۺϿ����⣬Ҫ��ѧ�����з����ͽ��������������ѶȲ���
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | �� | �ۢ� | ��� | �� | �ۢݢޢ� |
��3����������ǿ����ʣ�����ȫ���룬���뷽��ʽΪ��Al2��SO4��3=2Al3++3SO42-��17.1gAl2��SO4��3����ˮ���250mL��Һ��SO42-��������Ϊ
 =9.03��1022��SO42-�����ʵ���Ũ��c=
=9.03��1022��SO42-�����ʵ���Ũ��c=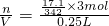 =0.6mol/L���ʴ�Ϊ��9.03��1022��0.6mol/L��
=0.6mol/L���ʴ�Ϊ��9.03��1022��0.6mol/L����4�������Ķ�����̼���Ժ�ǿ������������Ӧ����̼�ᱵ��ˮ����Ba��OH��2+CO2=BaCO3��+H2O��
�ʴ�Ϊ��Ba��OH��2+CO2=BaCO3��+H2O��
��������1���������ʵķ�����Լ������������ж��������������
��2����ͼ�֮����Է����кͷ�Ӧ�����κ�ˮ��
��3�����ݵ��뷽��ʽ����д����֪ʶ���ش𣬸��ݹ�ʽN=nNA=
 NA�Լ�c=
NA�Լ�c= ������ش�
������ش���4�������Ķ�����̼���Ժ�ǿ�Ӧ����̼���κ�ˮ��
������������һ���������ʵķ��ࡢ���ʵ����ļ����Լ����ʵ����ʷ���֪ʶ���ۺϿ����⣬Ҫ��ѧ�����з����ͽ��������������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ