��Ŀ����
| |||||||||||||||
������
(1) |
|
(2) |
|
(3) |
������m��12n ������m��12n ������m��12n |

 ��������������������ϵ�д�
��������������������ϵ�д���14�֣�ÿ��2�֣� I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2 (g)
(g)  2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
��1��Ϊ����߰����IJ��ʣ�����ѡ��ķ����� _______������ţ���ѡ�۷֣���
�� �����¶� �� ����ѹǿ �� ʹ�ô��� �� ��ʱ�����NH3
��2�������ܱ������н��еĿ��淴Ӧ��N2(g)+3H2(g) 2NH3(g)��������������£�
2NH3(g)��������������£�
˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬____________________________________��
��N2������Ӧ������NH3���淴Ӧ���ʵ�1/2
���ں��������£���������ѹǿ���ֲ���
��N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
�ܵ�λʱ����ÿ����3mol H2��ͬʱ��2mol NH3����
��3mol N-N�����ѣ�ͬʱ��6mol N-H������
��3��һ�������£�NH3�ڹ̶�������ܱ������з����ֽⷴӦ����H��0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ����ǣߣߣߣ�____________��ѡ����ţ���
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ����NH3�����ʵ��� |
| y | NH3�����ʵ��� | ƽ�ⳣ��K | NH3��ת���� | ���������ʵ����ܺ� |
II����1����������Һ�У������(KIO3)���������ƿɷ������·�Ӧ��
2IO3����5SO32����2H��===I2��5SO42����H2O
���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ��
| | 0.01mol��L��1 KIO3������Һ(������)�����/mL | 0.01mol��L��1 Na2SO3��Һ�����/mL | H2O����� /mL | ʵ�� �¶� /�� | ��Һ������ɫʱ����ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | --------- |
| ʵ��2 | 5 | 5 | 40 | 25 | ---------- |
| ʵ��3 | 5 | 5 | V2 | 0 | ----------- |
________________________________________________��
����V1=___________mL.
��2�����淴ӦC(s)+H2O(g)
 H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ����
H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ���� ��ʱ��t�Ĺ�ϵ����ͼ��
��ʱ��t�Ĺ�ϵ����ͼ����ͼ��t4��t6��ʱ����ƽ���ƶ�������������
 ��
����ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ���� ��

��14�֣�ÿ��2�֣�
I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2(g)
 2NH3(g) ��H= -92.4 kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H= -92.4 kJ/mol �ݴ˻ش��������⣺
��1��Ϊ����߰����IJ��ʣ�����ѡ��ķ����� _______ ������ţ���ѡ�۷֣���
�� �����¶� �� ����ѹǿ �� ʹ�ô��� �� ��ʱ�����NH3
��2�������ܱ������н��еĿ��淴Ӧ��N2(g)+3H2(g) 2NH3(g)��������������£�
2NH3(g)��������������£�
˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬____________________________________��
��N2������Ӧ������NH3���淴Ӧ���ʵ�1/2
���ں��������£���������ѹǿ���ֲ���
��N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
�ܵ�λʱ����ÿ����3mol H2��ͬʱ��2mol NH3����
��3mol N-N�����ѣ�ͬʱ��6mol N-H������
��3��һ�������£�NH3�ڹ̶�������ܱ������з����ֽⷴӦ����H��0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ����ǣߣߣߣ�____________��ѡ����ţ���
|
ѡ�� |
a |
b |
c |
d |
|
x |
�¶� |
�¶� |
����H2�����ʵ��� |
����NH3�����ʵ��� |
|
y |
NH3�����ʵ��� |
ƽ�ⳣ��K |
NH3��ת���� |
���������ʵ����ܺ� |
II����1����������Һ�У������(KIO3)���������ƿɷ������·�Ӧ��
2IO3����5SO32����2H��===I2��5SO42����H2O
���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ��
|
|
0.01mol��L��1 KIO3������Һ(������)�����/mL |
0.01mol��L��1 Na2SO3��Һ�����/mL |
H2O����� /mL |
ʵ�� �¶� /�� |
��Һ������ɫʱ����ʱ��/s |
|
ʵ��1 |
5 |
V1 |
35 |
25 |
--------- |
|
ʵ��2 |
5 |
5 |
40 |
25 |
---------- |
|
ʵ��3 |
5 |
5 |
V2 |
0 |
----------- |
��ʵ���Ŀ����_______________________________________________________________
________________________________________________��
����V1=___________mL.
��2�����淴ӦC(s)+H2O(g)  H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ����
H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ���� ��ʱ��t�Ĺ�ϵ����ͼ��
��ʱ��t�Ĺ�ϵ����ͼ��
��ͼ��t4��t6��ʱ����ƽ���ƶ������������� ��
��ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ���� ��
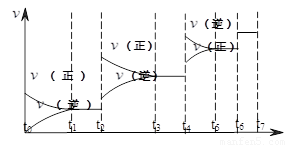

 2CO(g)
��H��0���ﵽƽ��״̬���ֽ������²����������߷�Ӧ��ϵ���¶ȣ������ӷ�Ӧ��C������������С��Ӧ��ϵ��������ܼ�С��ϵ��CO������������ʩ��һ����ʹ��Ӧ������Ӧ���������ӿ���� �� ��
2CO(g)
��H��0���ﵽƽ��״̬���ֽ������²����������߷�Ӧ��ϵ���¶ȣ������ӷ�Ӧ��C������������С��Ӧ��ϵ��������ܼ�С��ϵ��CO������������ʩ��һ����ʹ��Ӧ������Ӧ���������ӿ���� �� �� 2CO(g) ��H��0���ﵽƽ��״̬���������²�����
2CO(g) ��H��0���ﵽƽ��״̬���������²�����