��Ŀ����
I ��6�֣� A��B��C�����ձ��зֱ�ʢ��200mL��ͬ���ʵ���Ũ�ȵ�ϡ����

��1���ֱ�д������װ������Ƭ���淢����Ӧ�����ӷ���ʽ��
A ��B ��C ��
��2��һ��ʱ��������ձ��е�����ǡ��ȫ�������ģ�C�в�����3.36L����״�������壬����ԭϡ������Һ�����ʵ���Ũ��= mol��L-1����ʱ�������ձ���Һ�������ɴ�С��˳��Ϊ�� ����д��ţ� ��
��3���Ƚ�A��B��C��������ʴ�����ʣ��ɿ쵽����˳���� ����д��ţ���
��7�֣�ij���е�λ���õ绯ѧԭ����SO2���Ʊ����ᣬװ������ͼ������ij�ִ������缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���

(1)ͨ��SO2�ĵ缫Ϊ________������缫��ӦʽΪ______________________���˵缫��pH________(�������С�����䡱)��
(2)�������Һ�е�H��ͨ������Ĥ________(�� ���������ҡ�����)�ƶ���ͨ�������ĵ缫��ӦʽΪ________________________________��
��13�֣� I ��1��A Fe+2H+=Fe2++H2����B Fe-2e- ="==" Fe2+ C 2H+ +2e- === H2��
��2��0.75 mol��L-1�� C> A= B ��3���ɿ쵽����˳���� BAC ����д��ţ���
II (1)���� SO2��2e����2H2O===SO��4H����2�֣��� ��С
(2) ���ҡ� O2��4e����4H��===2H2O ��2�֣� ������ÿ��1�֣�
��������
���������I ��1������װ��ͼ���ص��֪��A�����Ļ�ѧ��ʴ����Ӧ�����ӷ���ʽ��Fe+2H+��Fe2++H2����B��C����ԭ��أ�����B�����Ǹ������缫��Ӧʽ��Fe-2e-��Fe2+��C��������������Һ�е������ӷŵ磬�缫��Ӧʽ��2H+ +2e-��H2����
��2�����������ʵ�����3.36L��22.4L/mol��0.15mol���������ԭ���غ��֪��ϡ�����Ũ����0.15mol��0.2L��0.75mol/L��п��Ħ�������������ģ����������ձ���Һ�������ɴ�С��˳��ΪC��A��B��
��3��A���ǻ�ѧ��ʴ��B�����������ⸯʴ��C������������������������������A��B��C��������ʴ�����ʣ��ɿ쵽����˳����BAC��
II (1)ԭ����нϻ��õĽ����Ǹ�����ʧȥ���ӣ�����������Ӧ�����Ӿ����ߴ��ݵ�������������Һ�е��������������ƶ��������õ����ӣ�������ԭ��Ӧ���ڷ�Ӧ��SO2ʧȥ���ӣ�����ͨ��SO2�ĵ缫Ϊ��������缫��ӦʽΪO2��2e����2H2O===SO��4H������˴˵缫��pH��С��
��2����Һ�е��������������ƶ����������Һ�е�H��ͨ������Ĥ�����ƶ���ͨ�����������������缫��Ӧʽ��O2��4e����4H��===2H2O��
���㣺��������ĵ绯ѧ��ʴ��������ԭ��ص�Ӧ��Ӧ�á��жϺͼ��������缫��Ӧʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ������������������ѵ�������������ѧ����������������Ӧ������������Ĺؼ���ע�����ԭ��صĹ���ԭ����Ȼ��������������ü��ɡ�

 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�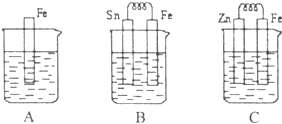 I��A��B��C�����ձ��зֱ�ʢ����ͬ���ʵ���Ũ�ȵ�ϡ���ᣮ
I��A��B��C�����ձ��зֱ�ʢ����ͬ���ʵ���Ũ�ȵ�ϡ���ᣮ


