��Ŀ����
��������ʮ�����ʣ���Һ̬HCl ��NaHCO3 ��NaCl��Һ ��CO2 �����Ǿ��� ��Ba(OH)2 �ߺ��ɫ�������������� ��NH3��H2O ����� ��Al2(SO4)3
��1������ʮ��������������������ˮ��Һ�пɷ�����Ӧ�����ӷ���ʽΪ��H����OH��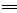 H2O���÷�Ӧ�Ļ�ѧ����ʽΪ ��
H2O���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2������ˮ�еĵ��뷽��ʽΪ ��
��3��θҺ�к������ᣬθ�������˳���θ�����ĵĸо���������ˮ������������С�մ�NaHCO3����������θ����࣬��д���䷴Ӧ�����ӷ���ʽ�� ���������ͬʱ��θ����Ϊ��θ�ڴ��ף����ܷ���С�մ�ʱ����ú�Al(OH)3��θҩ����θ��ƽ��������θ�ᷴӦ�����ӷ���ʽ��
��4����Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
��д����Ӧ�����ӷ���ʽ ��
27����1��Ba��OH��2+2HCl=BaCl2+2H2O����2�� Al2��SO4��3=2Al3++3SO42-�� ��3��HCO3-+H+=H2O+CO2����Al��OH��3+3H+=Al3++3H2O����4��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O
��������
�����������1�������������ڿ�����ǿ����Ȼ������ڿ�����ǿ�ᣬ���߷�Ӧ�Ļ�ѧ����ʽΪ��Ba��OH��2+2HCl=BaCl2+2H2O�����ӷ���ʽ���Ա�ʾΪ��H++OH-�TH2O����Ϊ��Ba��OH��2+2HCl=BaCl2+2H2O����2����������ǿ����ʣ���ˮ����ȫ���룬���뷽��ʽΪ��Al2��SO4��3=2Al3++3SO42-����Ϊ��Al2��SO4��3=2Al3++3SO42-����3��С�մ������ᷴӦ�����ӷ���ʽΪ��HCO3-+H+=H2O+CO2��������������������Ӧ�����ӷ���ʽΪ��Al��OH��3+3H+=Al3++3H2O����Ϊ��HCO3-+H+=H2O+CO2����Al��OH��3+3H+=Al3++3H2O����4������Ba��OH��2��Һ����μ���ϡ���ᣬ��Ӧ�������ᱵ��ˮ�������ӷ�ӦΪ��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O����Ϊ��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O��
���㣺�������ӷ���ʽ�����뷽��ʽ����д��

��15�֣�Ϊ��̽��H2O2��H2SO3��Br2�����Ե����ǿ�����������ʵ�飨�г���������ȥ������ش��������⣺

��1������A������_________����������___________��
��2��������B�μ�Һ�岢����Ҫ������c��ԭ����____________________________��
��3��ʵ���¼���£��벹ȫ�հף���
���� | ʵ����� | ʵ������ | ʵ����� |
�� | ����a����μ���H2SO3��Һ������ | ________________ | __________________________ |
�� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | __________________________ |
��4��������У���ʼʱ��ɫ�����Ա仯��ԭ���ǣ�д��һ����_______________________��
������з�Ӧ�����ӷ���ʽ_________________________________________________��
���������Ҫ��Ӧ�����ӷ���ʽ_____________________________________________��

 mol��L��1
mol��L��1