��Ŀ����
��15�֣�A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��DԪ��ͬ���壬B��CԪ��ͬ���ڣ���A��B��C��D�е�����Ԫ�ؿ��γ�ԭ�Ӹ�����Ϊ1:1�Ķ��ֻ�����ס��ҡ�������Ϊ���е����֣����ǵ�Ԫ��������±���ʾ��

�����£�������Ϊ���壬�ܶ���С�ڿ�����������ΪҺ�壻�����ʺͶ�����Ϊ�����Ҷ�Ϊ���ӻ��������д���пհף�
��1��DԪ���γɵļ����ӵĽṹʾ��ͼΪ �������ʵĻ�ѧʽΪ �����������������������ӵĸ���֮��Ϊ ��
��2������״����5.6L��������ȫȼ�շų�������ΪQKJ����д����ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ ��
��3���о����������ʾ��������ԣ�����������ˮ�еĵ��뷽��ʽΪ ��
��4��B��C����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��2���γɻ������죬A��C��D����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��1���γɻ����Z�������찴���ʵ���֮��Ϊ2:1��ȫ��Ӧ�����Һ�и�����Ũ�ȵĴ�С��ϵΪ ��
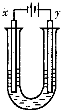
��5��ijͬѧ�����һ���Խṹ��ʽ��BA3��CA����Ϊȼ�ϵĵ�أ����øõ�ص��200mLһ��Ũ��NaCl��CuSO4�����Һ����װ������ͼ��

��д������ͨ���������һ���ĵ缫��Ӧʽ ��
�������Ϣ���������������������ʱ��仯�Ĺ�ϵ������ͼ��ʾ����������ѻ���ɱ�״���µ��������д����t1��ʯī�缫�ϵĵ缫��Ӧʽ ����t2ʱ������Һ��pHԼΪ ��

��15�֣�
��1�� NaH
1:2
(ÿ��1��)
NaH
1:2
(ÿ��1��)
��2�� CO(g)+1/2O2(g)= CO2 (g) ����H=-4akJ/mol (2��)
��3��H2O2
 H++HO2-��(HO2-
H++HO2-��(HO2-  H+
+O22-���Բ�д�ڶ�������) (2��)
H+
+O22-���Բ�д�ڶ�������) (2��)
��4��c(Na+)��c(CO32-)��c(OH-)��c(HCO3-)��c(H+) (2��)
��5���� CH3OH - 6e��+ 8OH�� = CO32��+ 6H2O ��2�֣�
�� 4OH�� - 4e�� = O2��+ 2H2O (��2 H2O - 4e�� = O2��+4 H+-�� ��2�֣�
1 �������𰸾��ɣ� ��2�֣���
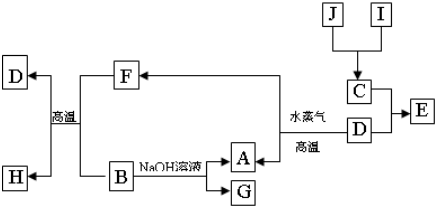
����������

 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ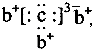 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
 Al��OH��3+OH-
Al��OH��3+OH-