��Ŀ����
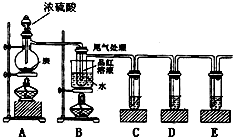
ijͬѧΪ�˼���Ũ������ľ̿���ڼ��������·�Ӧ����������������ѡ������ͼ��ʾʵ��װ�á�
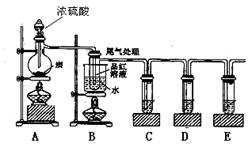
��1��д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
��2����֤����ˮ���ɣ���Ҫ��A��B֮���װʢ�������� __���ѧʽ������ĸ����
��3��C������KMnO4��Һ��������______________________________________��
��4��D���õ�Ʒ����Һ������������ ����������������������
��5��E�г��ֵ����������������������������������� ��������Ӧ�����ӷ���ʽ������������������������������������������������ ��
(6)װ��B����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������_______������
___________________________________________________________________________��
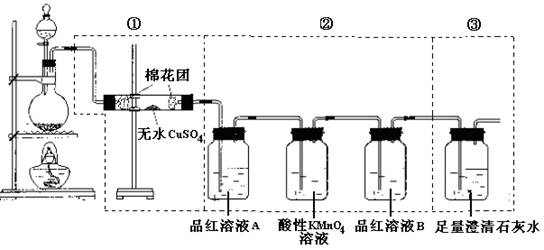
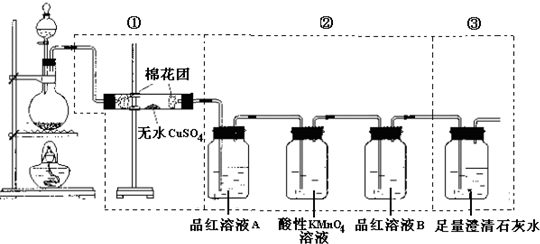
��1�� C+ 2H2SO4(Ũ)����![]() ��CO2��+ 2SO2��+ 2H2O
��CO2��+ 2SO2��+ 2H2O
��2��CUSO4�� �� 3����ȥ��������е�SO2
��4����SO2�Ƿ����
��5�����ְ�ɫ������ CO2 + Ca2+ + 2OH- = CaCO3��+ H2O
�� (6)Ʒ����ɫ���ٵ�ȼB���ľƾ��Ƽ��ȣ������ɫ����֤��SO2Ư���п�����

 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O