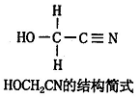
��Ŀ����
19����1�����Т�����ھƾ���Һ���ܴ�������ڵ��������Ƣ�ˮ����������������ʯ��ˮ������ �У������ε��ǣ�����ţ���ͬ���ܣ�����������������Ǣߣ��ܵ���� �Ǣ٢ݢޢ࣬���ڵ���ʵ��Ǣܢݣ���2��������������Ӧ��
a.2FeCl3+2KI=2FeCl2+2KCl+I2
b.2FeCl2+Cl2=2FeCl3
c.2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
����������Ӧ����������������ǿ�����Ĺ�ϵΪKMnO4��Cl2 ��FeCl3��
�ڷ�Ӧ c �У����������� HCl Ϊ 1.5mol����ת�Ƶĵ�������1.5NA����
��3��12.4g Na2X ���� 0.4mol Na��Na2X ��Ħ������Ϊ62g/mol��X �����ԭ������Ϊ16�������ʵĻ�ѧʽΪNa2O��
���� ��1������ָ�ɽ������ӻ�笠����������������ɵĻ����
�������������ܺͼӦ�����κ�ˮ�������������ķǽ��������������������
���ʵ���������Ǵ������ɵ��ӻ��������ƶ������ӣ�
���������ˮ��Һ�л�����״̬���ܵ���Ļ����
��2�����������������Դ�����������ݴ˷���������Ӧ�еĹ�ϵ�õ���
��2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O����Ӧ�б�����10molHCl����ת��10mol���㣻
��3��12.4��Na2X�к���0.4molNa+������Na2X����ɼ����Na2X�����ʵ�����Ħ������������Ħ����������Է������Ĺ�ϵ�����X�����ԭ������������Na2X���ԭ��������ȷ������ʽ��
��� �⣺��1���ܴ���������������̼���������ɵĻ���������Σ�
�������������������Ʒ�Ӧ���������ƺ�ˮ���������������
����������ڵ��������ƺ͢����ʯ��ˮ���������ƶ������ӣ��ܹ����磻��ˮ���������ɵ����ܹ����磻
�ܴ�������ڵ���������ˮ��Һ�л�����״̬���ܵ���Ļ�������ڵ���ʣ�
�ʴ�Ϊ���ܣ��ߣ��٢ݢޢࣻ�ܢݣ�
��2�����������������Դ������������
a.2FeCl3+2KI�T2FeCl2+2KCl+I2��������FeCl3 ����������I2��������FeCl3 ��I2��
b.2FeCl2+Cl2�T2FeCl3��������Cl2����������FeCl3��������Cl2��FeCl3��
c.2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O��������KMnO4����������Cl2��������KMnO4��Cl2��
������������ǿ����ϵΪKMnO4��Cl2 ��FeCl3��
�ʴ�Ϊ��KMnO4��Cl2 ��FeCl3��
��2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O����Ӧ�б�����10molHCl����ת��10mol������������HClΪ1.5mol����ת�Ƶĵ�������1.5NA��
�ʴ�Ϊ��1.5NA��
��3��12.4��Na2X�к���0.4molNa+��Na2X�����ʵ���Ϊ��n��Na2X��=$\frac{1}{2}$n��Na+��=0.4mol��$\frac{1}{2}$=0.2mol��
Na2X��Ħ������Ϊ��M��Na2X��=$\frac{12.4g}{0.2mol}$=62g/mol��
Ħ����������ֵ�ϵ�������Է�����������Na2X�����ԭ����Ϊ62��
��ԭ�ӵ����ԭ��������23������X�����ԭ��������62-23��2=16��
XΪ��ԭ�ӣ������ʵĻ�ѧʽΪNa2O��
�ʴ�Ϊ��62g/mol��16��Na2O��
���� ������Ҫ���������ʵķ��ࡢ������ԭ��Ӧ�����ʵ����ļ��㣬��Ŀ�ѶȲ���ע�������غ㶨�ɡ�Ħ����������ֵ�ϵ�������Է��������ȸ�������ã�������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����

| A�� | K+��Fe2+��NO3-��Cl- | B�� | Na+��Fe3+��SCN-��SO42- | ||
| C�� | K+��Na+��Cl-��AlO2- | D�� | Al3+��Na+��C1-��SO42- |
| A�� | 1 mol�κ�����������ԼΪ22.4L | |
| B�� | ��1L 10 mol/L��Ũ������9Lˮ���ʱ���ʵ���Ũ��Ϊ1 mol/L | |
| C�� | ��22.4L�Ȼ�����������ˮ���1L��Һʱ���ʵ���Ũ��Ϊ1 mol/L | |
| D�� | ��״���£�22.4L���κ���������ʵ�������1 mol |
 ��
��