��Ŀ����
��֪2Al+2NaOH+2H2O��2NaAlO2+3H2�����÷�Ӧ���й���������������ȷ���ǣ�NA��ʾ�����ӵ�������
- A.ÿ����0.3 mol H2������ԭ��ˮ������ĿΪ0.6 NA
- B.����2.7 g Al�μӷ�Ӧʱ��ת�Ƶĵ�����ĿΪ0.3 NA
- C.ÿ����6.72 L��H2����Һ��AlO2-����Ŀ������0.2 NA
- D.��Һ��ÿ����0.1 mol��AlO2-��Na+����Ŀ������0.1 NA
B
�������÷�Ӧ����ʧ��������ԭ������Ԫ�صõ������������������������ƺ�ˮ�����������ٸ��ݸ�����֮��Ĺ�ϵʽ���㣬ע������������ˮ�⣮
��𣺸÷�Ӧ����ʧ��������ԭ������Ԫ�صõ������������������������ƺ�ˮ����������
A�����ݷ���ʽ֪��ÿ����3 mol H2������ԭ��ˮ������ĿΪ2NA������ÿ����0.3 mol H2������ԭ��ˮ������ĿΪ0.2 NA����A����
B�����ݷ���ʽ֪������2.7 g Al�μӷ�Ӧʱ��ת�Ƶĵ�����Ŀ=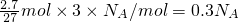 ����B��ȷ��
����B��ȷ��
C��ÿ����6.72 L��H2��������ƫ�����Ƶ����ʵ���=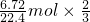 =0.2mol����Ϊ��Һ��AlO2-��ˮ�⣬��Һ��Һ��AlO2-����Ŀ������С��0.2 NA����C����
=0.2mol����Ϊ��Һ��AlO2-��ˮ�⣬��Һ��Һ��AlO2-����Ŀ������С��0.2 NA����C����
D��ƫ�����ƻ�ѧʽ��Na+��AlO2-�ĸ���֮����1��1��������Һ�У�AlO2-��ˮ���Na+��ˮ�⣬����Na+��������AlO2-������������Һ��ÿ����0.1 mol��AlO2-��Na+����Ŀ����������0.1 NA����D����
��ѡB��
���������⿼��������ԭ��Ӧ����ȷԪ�ػ��ϼ��ǽⱾ��ؼ���ע��÷�Ӧ��ˮ����Ӧ��ܶ�ѧ��������©��ˮ�μӷ�Ӧ�����´���Ϊ�״��㣮
�������÷�Ӧ����ʧ��������ԭ������Ԫ�صõ������������������������ƺ�ˮ�����������ٸ��ݸ�����֮��Ĺ�ϵʽ���㣬ע������������ˮ�⣮
��𣺸÷�Ӧ����ʧ��������ԭ������Ԫ�صõ������������������������ƺ�ˮ����������
A�����ݷ���ʽ֪��ÿ����3 mol H2������ԭ��ˮ������ĿΪ2NA������ÿ����0.3 mol H2������ԭ��ˮ������ĿΪ0.2 NA����A����
B�����ݷ���ʽ֪������2.7 g Al�μӷ�Ӧʱ��ת�Ƶĵ�����Ŀ=
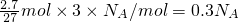 ����B��ȷ��
����B��ȷ��C��ÿ����6.72 L��H2��������ƫ�����Ƶ����ʵ���=
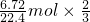 =0.2mol����Ϊ��Һ��AlO2-��ˮ�⣬��Һ��Һ��AlO2-����Ŀ������С��0.2 NA����C����
=0.2mol����Ϊ��Һ��AlO2-��ˮ�⣬��Һ��Һ��AlO2-����Ŀ������С��0.2 NA����C����D��ƫ�����ƻ�ѧʽ��Na+��AlO2-�ĸ���֮����1��1��������Һ�У�AlO2-��ˮ���Na+��ˮ�⣬����Na+��������AlO2-������������Һ��ÿ����0.1 mol��AlO2-��Na+����Ŀ����������0.1 NA����D����
��ѡB��
���������⿼��������ԭ��Ӧ����ȷԪ�ػ��ϼ��ǽⱾ��ؼ���ע��÷�Ӧ��ˮ����Ӧ��ܶ�ѧ��������©��ˮ�μӷ�Ӧ�����´���Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
��֪2Al+2NaOH+2H2O��2NaAlO2+3H2�������ڸ÷�Ӧ���й���������������ȷ���ǣ�NA��ʾ�����ӵ���������������
| A��ÿ����0.6mol H2������ԭ��ˮ������ĿΪ1.2NA | B����2.7g Al�μӷ�Ӧʱ��ת�Ƶĵ�����ĿΪ0.3NA | C����6.72L H2����ʱ����Ӧ��ת�Ƶĵ�����ĿΪ0.6NA | D����Һ��ÿ����0.1mol AlO2-��Na+����Ŀ������0.1NA |
 2NaAlO2��3H2���÷�Ӧ���й���������������ȷ���ǣ�NA��ʾ�����ӵ�������
2NaAlO2��3H2���÷�Ӧ���й���������������ȷ���ǣ�NA��ʾ�����ӵ�������