��Ŀ����
A��B���Ƿ����廯���1 mol Aˮ��õ�1 mol B��1 mol���ᣮA��B��Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����B��̼����Ԫ���������ٷֺ���Ϊ65.2��(����������Ϊ65.2��)��A����Һ�������ԣ�����ʹFeCl3��Һ��ɫ��(1)A��B����Է�������֮��Ϊ________��
(2)1��B������Ӧ����________����ԭ�ӣ�
(3)A�ķ���ʽΪ________��
(4)B���ܵ����ֽṹ��ʽΪ��________��________��________��
�𰸣�
������
������
(1)42��(2)3��(3)C9H8O4��(4)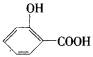 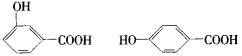
������(1)������ã�A+H2O��B+CH3COOH����A-B=42��(2)����֪A�����Ȼ�����B��Ҳ���Ȼ�����B���д�A��ˮ��������ǻ�����B����3����ԭ�ӣ�(3)��B��C��H����������֪��Ar(B)=
|

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ��
�� ����д���֣�
����д���֣�

