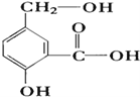
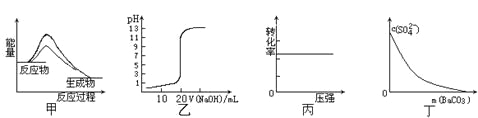
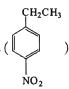
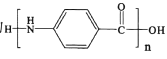
��Ŀ����
����Ŀ������ͼ��ʾװ���н��а��Ĵ�����ʵ�飺������ƿ�ڵ�Ũ��ˮ�в���ͨ������������ȵIJ�˿����ƿ�в��ӽ�Һ�档��Ӧ�����У��ɹ۲쵽ƿ���к���ɫ�����������˿ʼ�ձ��ֺ��ȡ������й�˵���������(����)

A. ��Ӧ����Һ�к���NO3-
B. ��Ӧ����Һ��c(H��)����
C. ʵ��������л��Ϸ�Ӧ����
D. ʵ�������NH3H2O�ĵ��볣�������ܷ����仯
���𰸡�D
��������A����Ӧ�����������ᣬ������������������ӣ�A��ȷ��B����Ӧ�������ᣬ������ǿ�ᣬ���Է�Ӧ����Һ��c��H+������pHֵ��С��B��ȷ��C.2NO+O2=2NO2��Ϊ���Ϸ�Ӧ��C��ȷ��D������������Ӧ���ȷ�Ӧ����Һ�¶����ߣ�ʵ�������NH3H2O�ĵ��볣���仯��D����ѡD��

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�
�����Ŀ