��Ŀ����
�±�ΪԪ�����ڱ���һ���֣���ش��й�����

��1���ݺ͢��Ԫ�ط�����______��_______��
��2����������õĽ�����________���ǽ�������ǿ��Ԫ����_________��(��дԪ�ط���)
��3���������γ��������������Ԫ����_______��(��дԪ�ط���)�ֱ�д����Ԫ�ص�����������ޡ��������������Ӧˮ���ﷴӦ�����ӷ���ʽ��_____________________��____________________��
��4���ݺ͢��γ�ֻ��һ������ԭ�ӵĻ�����a��a�ķ���ʽ��______��a���ӵ�����ṹ��__________��
a�Ǻ�______��������ԡ��Ǽ��ԡ�����______���ӣ�������ԡ��Ǽ��ԡ���
��5���ۺܱ͢Ƚϣ��縺�Խϴ����______����һ�����ܽϴ����______(��Ԫ�ط���)�����һ�����ܽϴ��ԭ����_________________________________��
��2����������õĽ�����________���ǽ�������ǿ��Ԫ����_________��(��дԪ�ط���)
��3���������γ��������������Ԫ����_______��(��дԪ�ط���)�ֱ�д����Ԫ�ص�����������ޡ��������������Ӧˮ���ﷴӦ�����ӷ���ʽ��_____________________��____________________��
��4���ݺ͢��γ�ֻ��һ������ԭ�ӵĻ�����a��a�ķ���ʽ��______��a���ӵ�����ṹ��__________��
a�Ǻ�______��������ԡ��Ǽ��ԡ�����______���ӣ�������ԡ��Ǽ��ԡ���
��5���ۺܱ͢Ƚϣ��縺�Խϴ����______����һ�����ܽϴ����______(��Ԫ�ط���)�����һ�����ܽϴ��ԭ����_________________________________��
��1��Si��Br
��2��K��F
��3��Al��Al(OH)3+3H+=Al3++3H2O��Al(OH)3+OH-=AlO2-+2H2O
��4��SiCl4���������壻���ԣ��Ǽ���
��5��Mg��Al��þ�����������Ų�Ϊ3s2������ȫ�����ṹ��ԭ�ӵ������ϵͣ�����Խϴ�ĵ�һ�����ܣ����������������Ų�3s23p1������ȫ����Ҳ���ǰ������ȫ�գ�����ԭ�ӵ������ϸߣ���ʧȥһ������ʱ�ִﵽ��3s2��ȫ�����ṹ�����������Խ�С�ĵ�һ������
��2��K��F
��3��Al��Al(OH)3+3H+=Al3++3H2O��Al(OH)3+OH-=AlO2-+2H2O
��4��SiCl4���������壻���ԣ��Ǽ���
��5��Mg��Al��þ�����������Ų�Ϊ3s2������ȫ�����ṹ��ԭ�ӵ������ϵͣ�����Խϴ�ĵ�һ�����ܣ����������������Ų�3s23p1������ȫ����Ҳ���ǰ������ȫ�գ�����ԭ�ӵ������ϸߣ���ʧȥһ������ʱ�ִﵽ��3s2��ȫ�����ṹ�����������Խ�С�ĵ�һ������

��ϰ��ϵ�д�
�����Ŀ








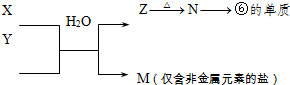
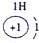
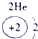
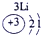
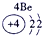
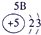
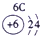
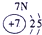
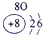
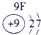
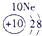
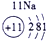





 ��ʾ����
��ʾ����