��Ŀ����
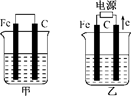
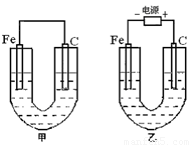
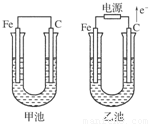
�ס������صĵ缫���϶���������̼��(����ͼ)����ش��������⣺

(1)�������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е�________�����ҳ��е�________����
�����ҳ��������ĵ缫��Ӧʽ��________________��
(2)�������о�ʢ�ű���NaCl��Һ����Ӧһ��ʱ���
��д���ҳ��з������ܷ�Ӧ�����ӷ���ʽ____________________��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
�����ҳ�ת��0.02 mol���Ӻ�ֹͣʵ�飬������Һ�������200 mL������Һ���Ⱥ��pH��________.
���к�ɫ�����������ǣ��׳��е�________�����ҳ��е�________����
�����ҳ��������ĵ缫��Ӧʽ��________________��
(2)�������о�ʢ�ű���NaCl��Һ����Ӧһ��ʱ���
��д���ҳ��з������ܷ�Ӧ�����ӷ���ʽ____________________��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
�����ҳ�ת��0.02 mol���Ӻ�ֹͣʵ�飬������Һ�������200 mL������Һ���Ⱥ��pH��________.
(1)��̼(C)����(Fe)����Cu2����2e��===Cu
(2)��2Cl����2H2O 2OH����H2����Cl2������5Cl2��I2��6H2O===10HCl��2HIO3����13
2OH����H2����Cl2������5Cl2��I2��6H2O===10HCl��2HIO3����13
(2)��2Cl����2H2O
 2OH����H2����Cl2������5Cl2��I2��6H2O===10HCl��2HIO3����13
2OH����H2����Cl2������5Cl2��I2��6H2O===10HCl��2HIO3����13 
��ϰ��ϵ�д�
�����Ŀ