��Ŀ����
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺
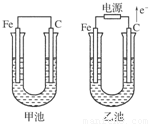
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5 ��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
(1)��̼��������4OH����4e��===2H2O��O2��
(2)��2Cl����2H2OCl2����H2����2OH��
��2H2O��O2��4e��===4OH����������Ӧ
��5Cl2��I2��6H2O===2HIO3��10HCl����13
����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�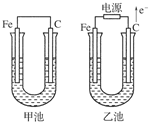
 Ϊ������ļ����������أ���ش�
Ϊ������ļ����������أ���ش�
 ______��
______��