��Ŀ����
��Ѫ�Σ������軯�أ�K4[Fe(CN)6] ��������ˮ���㷺����ʳ�����Ӽ������������ʳ���л�Ѫ�ε����ʹ����Ϊ10 mg��kg��1����Ѫ�ξ���ʱ�������400��ʱ��ֽ����ɾ綾���軯�ء��ش��������⣺
��1��д����̬Fe2+�ĺ�������Ų�ʽ_______________��K4[Fe(CN)6]��Fe2+��CN����������֮�����������______________��
��2��CN����̼ԭ�ӵ��ӻ���ʽΪ_____________��1molCN���к��Цм�����ĿΪ________��
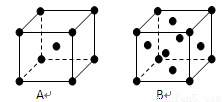
��3�������ء�ͭ�ľ���ľ����ṹ����ͼ�������ж϶�Ӧ��ͼ�����ء�ͭ���־��徧���н���ԭ�ӵ���λ��֮��Ϊ _______________ ��
��1��д����̬Fe2+�ĺ�������Ų�ʽ_______________��K4[Fe(CN)6]��Fe2+��CN����������֮�����������______________��
��2��CN����̼ԭ�ӵ��ӻ���ʽΪ_____________��1molCN���к��Цм�����ĿΪ________��
��3�������ء�ͭ�ľ���ľ����ṹ����ͼ�������ж϶�Ӧ��ͼ�����ء�ͭ���־��徧���н���ԭ�ӵ���λ��֮��Ϊ _______________ ��

��4����Ѫ����Һ��ϡ�������ʱ������������ԭ��Ӧ�����������κ�һ����CN���ǵȵ��������̬�������Ӧ��ѧ����ʽΪ_______________ ��
��1��1s22s22p63s23p63d6 ��[Ar]3d6�����
��2��sp�ӻ���2NA��
��3��2��3
��4��K4Fe(CN)6 + 6H2SO4 + 6H2O 2K2SO4 + FeSO4+3(NH4)2SO4 +6CO��
2K2SO4 + FeSO4+3(NH4)2SO4 +6CO��
��2��sp�ӻ���2NA��
��3��2��3
��4��K4Fe(CN)6 + 6H2SO4 + 6H2O
 2K2SO4 + FeSO4+3(NH4)2SO4 +6CO��
2K2SO4 + FeSO4+3(NH4)2SO4 +6CO�� 
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ