��Ŀ����
����Ŀ�����������ԭ�Ӷѻ���ʽ�����������֣�������۲�ģ��(��ͼ)���ش��������⣺
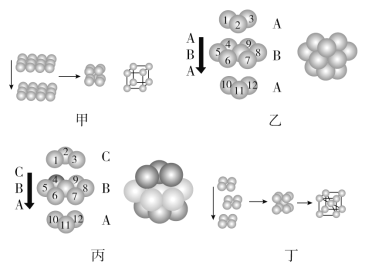
��1�����ֶѻ�ģ�͵Ķѻ�����������________��________��________��________��
��2���ѻ���ʽ�еĿռ�������Ϊ________��ֻ�н���________�������ֶѻ���ʽ��
��3������������ֶѻ���ʽ�н���ԭ�ӵ���λ��________(������ͬ����������ͬ��)�����еĿռ�������Ϊ________��
��4����ȡ���жѻ���ʽ�Ľ���ͨ����________(��д���ֽ���Ԫ�صķ���)��ÿ�������������е�ԭ����Ϊ________��
���𰸡��������ѻ� �������ܶѻ� �����������ܶѻ� ���������ѻ� 52% Po(��) ��ͬ 74% K��Na��Fe(��������) 2
��������
��1���������ѻ����������ò�Ľ���ԭ�����¶��룬�γɵľ�����1�������壬���������ÿ��������1������ԭ�ӣ���Ϊ�������ѻ���
�������ܶѻ��������������ܶѻ��������ܶѻ��������������ܶѻ���һ�������塭���������غϣ������ġ��������������غϣ������������ܶѻ����ġ��塢������ֱ��һ���������������غϣ�ͼ���Ķѻ���ʽ�ǽ������ò���ϲ����ԭ�������²����ԭ���γɵİ�Ѩ�У�ÿ����մ˶ѻ����γɵľ�����1�������壻
���������ѻ������������ÿ��������1��ԭ�ӣ�����������ĺ���1������ԭ�ӣ�
��2���������ѻ��Ŀռ���������ͣ��ռ�������Ϊ52%����ȡ���ֶѻ���ʽ��ֻ��Po��
��3���������ܶѻ�����λ��12���ռ�������74%�������������ܶѻ�����λ��12���ռ�������74%
��4�����������ѻ�����λ��8��ÿ�������к��н���ԭ�ӵĸ���Ϊ��1+8��![]() =2��
=2��
��1���Ķѻ���ʽ�ǽ������ò�Ľ���ԭ�����¶��룬�γɵľ�����1�������壬���������ÿ��������1������ԭ�ӣ���Ϊ�������ѻ����Һͱ��������ò�ԭ�ӵĶѻ���ʽ��������A�����A���3��ԭ����ɵ������η�����ͬ����Ϊ�������ܶѻ�������a���C���3��ԭ����ɵ������η����෴����Ϊ�����������ܶѻ���ͼ���Ķѻ���ʽ�ǽ������ò���ϲ����ԭ�������²����ԭ���γɵİ�Ѩ�У�ÿ����մ˶ѻ����γɵľ�����1�������壬���������ÿ��������1��ԭ�ӣ�����������ĺ���1������ԭ�ӣ���Ϊ���������ѻ���
�ʴ�Ϊ���������ѻ����������ܶѻ��������������ܶѻ������������ѻ���
��2���Ķѻ���ʽ�������ѻ����������ѻ��Ŀռ���������ͣ��ռ�������Ϊ52%����ȡ���ֶѻ���ʽ��ֻ��Po��
�ʴ�Ϊ��52%��Po��
��3���Һͱ����ֶѻ���ʽ�У�����ԭ�ӵ���λ����Ϊ12������ռ������ʾ�Ϊ74%��
�ʴ�Ϊ����ͬ��74%��
��4���������������ѻ�����ȡ���ֶѻ���ʽ�Ľ�����K��Na��Fe�ȣ��þ�̯�������ÿ�������к��н���ԭ�ӵĸ���Ϊ��1+8��![]() =2��
=2��
�ʴ�Ϊ��K��Na��Fe���������ɣ���2��