��Ŀ����
19�� ��ͼ�����ڱ��ж����ڵ�һ���֣�W��X��Y����Ԫ��ԭ�Ӻ��������֮�͵���X����������Xԭ�Ӻ�������������������ȣ����������в���ȷ���ǣ�������
��ͼ�����ڱ��ж����ڵ�һ���֣�W��X��Y����Ԫ��ԭ�Ӻ��������֮�͵���X����������Xԭ�Ӻ�������������������ȣ����������в���ȷ���ǣ�������| A�� | ����Ԫ�ص�ԭ�Ӱ뾶�Ĵ�С˳����W��Y��X | |
| B�� | W����������Ӧˮ�������ǿ���ԣ���̬�⻯���ˮ��Һ���������� | |
| C�� | XԪ�ص�������⻯���ˮ��Һ�������� | |
| D�� | YԪ�صĵ����Ƿǽ���������Ψһ�ܸ�ˮ�������ҷ�Ӧ�ĵ��� |
���� ͼ�����ڱ��ж����ڵ�һ���֣���֪W��Y���ڵڶ����ڣ�X���ڵ������ڣ���XԪ�ص�������Ϊa����W��������Ϊa-9��Y��������Ϊa-7��XԪ�ص�ԭ�Ӻ�����������������������X��������Ϊ2a��W��X��Y����Ԫ�ص�ԭ�Ӻ������������X������������a-9+a+a-7=2a����֮��a=16����XΪS��WΪN��YΪF���ݴ˽��
��� �⣺ͼ�����ڱ��ж����ڵ�һ���֣���֪W��Y���ڵڶ����ڣ�X���ڵ������ڣ���XԪ�ص�������Ϊa����W��������Ϊa-9��Y��������Ϊa-7��XԪ�ص�ԭ�Ӻ�����������������������X��������Ϊ2a��W��X��Y����Ԫ�ص�ԭ�Ӻ������������X������������a-9+a+a-7=2a����֮��a=16����XΪS��WΪN��YΪF��
A��ͬ�����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ԭ�Ӱ뾶Y��W��X����A����
B��WΪNԪ�أ�����������Ӧˮ����Ϊ���ᣬ����ǿ���ԣ����⻯��Ϊ������ˮ��Һ�������ԣ���B��ȷ��
C��XΪSԪ�أ�����������Ϊ��������������������ˮ�õ������ᡢ���ᣬ��Һ�����ԣ��⻯��Ϊ���⣬��ˮ��Һ�����ԣ���C��ȷ��
D���ǽ���������ֻ�з�����ˮ���ҷ�Ӧ����HF����������D��ȷ��
��ѡA��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã�����λ�ù�ϵȷ��ԭ��������ϵ�ǹؼ���ע������Ԫ�����ڱ��ṹ��ע���Ԫ�ػ�����֪ʶ�����գ��ѶȲ���

| A�� | ������и�Ԫ�ص������ٷֺ�������ͬ�������DZض���ͬϵ�� | |
| B�� | ��Է���������ͬ���ṹ��ͬ������һ����ͬ���칹�� | |
| C�� | �����ʵĺ������ͼ���Ի�÷����к��л�ѧ��������ŵ���Ϣ | |
| D�� | �Ӻ˴Ź�������ͼ������֪���л�������м��ֲ�ͬ���͵���ԭ�Ӽ����ǵ���Ŀ |
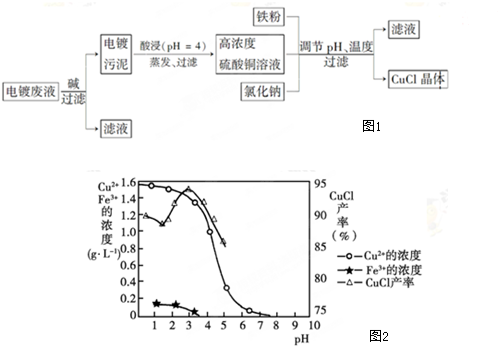
 �Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������
�Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ�������ĵ�ط�ӦʽΪAlMn2O4-3e-�TMn2O4+Al3+ | |
| B�� | �ŵ�ʱ��Al3+���ƶ� | |
| C�� | ���ʱ��Mn2O4�����Դ�ĸ������� | |
| D�� | ���ʱ��Al�缫�������� |
| A�� | ��Fe��NO3��2ϡ��Һ�м������Fe2++2H+�T3Fe2++H2�� | |
| B�� | ͭƬ����ϡ�����У�3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O | |
| C�� | ̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4++OH-�TNH3��+H2O | |
| D�� | AlCl3��Һ�м��������ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O |
| A�� | 120�� | B�� | 117.6�� | C�� | 116�� | D�� | 121�� |

| A�� | ��̬�⻯���ȶ��ԣ�R��Q | |
| B�� | Ԫ��T�����Ӱ뾶��Ԫ��R�����Ӱ뾶 | |
| C�� | ��wͬ�����ijԪ���γɵ�18���ӵ��⻯������м��м��Լ����зǼ��Լ� | |
| D�� | Q���γɶ��ֺ����� |
| A�� | ͭ��Ũ���ᷴӦ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O | |
| B�� | ̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| C�� | ������ͭ�������2H++Cu��OH��2=Cu2++2H2O | |
| D�� | ������������ϡ���3Fe2++4H++NO3-=3Fe3++NO��+2H2O |