��Ŀ����
�����������ѧ����ʽ(X��Y��Z��Ϊ��ֵ)�� ��C2H2(g)+H2(g)![]() C2H4(g)
C2H4(g)
��CH4(g)![]() H2(g)+
H2(g)+![]() C2H4(g)
C2H4(g)
��C(s)+2H2(g)![]() CH4(g)����H=-X kJ��mol-1
CH4(g)����H=-X kJ��mol-1
��C(s)+![]() H2(g)
H2(g)![]()
![]() C2H2(g)����H=-YkJ��mol-1
C2H2(g)����H=-YkJ��mol-1
��C(s)+H2(g)![]()
![]() C2H4(g)����H=-ZkJ��mol-1
C2H4(g)����H=-ZkJ��mol-1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж��ۡ���ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ����( )
A.X��Y��Z B.X��Z��Y C.Y��X��Z D.Z��X��Y
B
�����������⿼�黯ѧƽ����ƶ��ͷ�Ӧ�ȵļ��㣬�е��⡣���¶��½�ʱ����ʽƽ�������ƶ�����ʽƽ�������ƶ���˵���ٵ�����ӦΪ���ȷ�Ӧ(��H1��0)���ڵ�����ӦΪ���ȷ�Ӧ(��H2��0)����(��-��)��2���Եõ��٣��ʦ�H1=2[-Z kJ��mol(-Y kJ��mol)]��0����Y��Z���ɢ�-�ۿ��Եõ��ڣ��ʦ�H2=-Z kJ��mol-(-X kJ��mol)��0����X��Z��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�����������ѧ����ʽ��X��Y��Z��Ϊ��ֵ����
��C2H2��g��+H2��g��?C2H4��g��
��CH4��g��?H2��g��+
C2H4��g��
��C��s��+2H2��g��?CH4��g������H=-X kJ?mol-1
��C��s��+
H2��g��?
C2H2��g������H=-Y kJ?mol-1
��C��s��+H2��g��?
C2H4��g������H=-Z kJ?mol-1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ���ǣ�������
��C2H2��g��+H2��g��?C2H4��g��
��CH4��g��?H2��g��+
| 1 |
| 2 |
��C��s��+2H2��g��?CH4��g������H=-X kJ?mol-1
��C��s��+
| 1 |
| 2 |
| 1 |
| 2 |
��C��s��+H2��g��?
| 1 |
| 2 |
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ���ǣ�������
| A��X��y��Z |
| B��X��Z��Y |
| C��Y��X��Z |
| D��Z��X��Y |
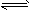 C2H4��g��
C2H4��g�� 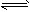 H2��g��+1/2C2H4��g��
H2��g��+1/2C2H4��g��  CH4��g������H= -X kJ��mol-1
CH4��g������H= -X kJ��mol-1 1/2C2H2��g������H= -Y kJ��mol-1
1/2C2H2��g������H= -Y kJ��mol-1 1/2C2H4��g����H= -ZkJ��mol-1
1/2C2H4��g����H= -ZkJ��mol-1