��Ŀ����
�����������ѧ����ʽ��x��Y��Z��Ϊ��ֵ����
��C2H2��g��+H2��g��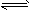 C2H4��g��
C2H4��g��
��CH4��g��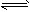 H2��g��+1/2C2H4��g��
H2��g��+1/2C2H4��g��
��C��s��+2H2��g�� CH4��g������H= -X kJ��mol-1
CH4��g������H= -X kJ��mol-1
��C��s��+1/2H2��g�� 1/2C2H2��g������H= -Y kJ��mol-1
1/2C2H2��g������H= -Y kJ��mol-1
��C��s��+H2��g�� 1/2C2H4��g����H= -ZkJ��mol-1
1/2C2H4��g����H= -ZkJ��mol-1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ����
��C2H2��g��+H2��g��
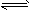 C2H4��g��
C2H4��g�� ��CH4��g��
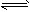 H2��g��+1/2C2H4��g��
H2��g��+1/2C2H4��g�� ��C��s��+2H2��g��
 CH4��g������H= -X kJ��mol-1
CH4��g������H= -X kJ��mol-1��C��s��+1/2H2��g��
 1/2C2H2��g������H= -Y kJ��mol-1
1/2C2H2��g������H= -Y kJ��mol-1��C��s��+H2��g��
 1/2C2H4��g����H= -ZkJ��mol-1
1/2C2H4��g����H= -ZkJ��mol-1 ���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ����
[ ]
A��X>Y>Z
B��X>Z>Y
C��Y>X>Z
D��Z>X>Y
B��X>Z>Y
C��Y>X>Z
D��Z>X>Y
B

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����������ѧ����ʽ��X��Y��Z��Ϊ��ֵ����
��C2H2��g��+H2��g��?C2H4��g��
��CH4��g��?H2��g��+
C2H4��g��
��C��s��+2H2��g��?CH4��g������H=-X kJ?mol-1
��C��s��+
H2��g��?
C2H2��g������H=-Y kJ?mol-1
��C��s��+H2��g��?
C2H4��g������H=-Z kJ?mol-1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ���ǣ�������
��C2H2��g��+H2��g��?C2H4��g��
��CH4��g��?H2��g��+
| 1 |
| 2 |
��C��s��+2H2��g��?CH4��g������H=-X kJ?mol-1
��C��s��+
| 1 |
| 2 |
| 1 |
| 2 |
��C��s��+H2��g��?
| 1 |
| 2 |
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж���-��ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ���ǣ�������
| A��X��y��Z |
| B��X��Z��Y |
| C��Y��X��Z |
| D��Z��X��Y |