��Ŀ����
��֪��ǰ18��Ԫ����ɵ����ַ���A��B��C��D��E����������ԭ�Ӻ���Ŀ����Ϊ2��2��3��3��4��5������A��B������18�����ӣ�C��D��E������10�����ӣ���ش���1��DΪ______���ӣ�ѡ��ԡ��Ǽ��ԣ��������ʽΪ______��
��2��EΪ______�ռ乹�ͣ��û������ڹ�̬ʱΪ______���壬C�к��Ļ�ѧ��������______��ѡ��Լ����Ǽ��Լ������Ӽ���
��3����B����ͨ��ij��ɫ��Һ�У���Һ���ֺ�ɫ���dz�������Ӧ�Ļ�ѧ����ʽΪ______��
��4����A��D�ɰ�1��1�����������ӻ�����X��XΪ______���ѧʽ��
���𰸡���������ǰ18��Ԫ����ɵ����ַ���A��B��C��D��E��������ԭ�Ӻ���Ŀ����Ϊ2��3��3��4��5������A��B������18�����ӣ�C��D��E������10�����ӣ���A��B��C��D��E�ֱ���HCl��H2S��H2O��NH3��CH4��
��1��������ṹȷ�����Ӽ��ԣ�����ṹ�Գ������ڷǼ��Է��ӣ��������ڼ��Է��ӣ�
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ��ɷ��ӹ��ɵľ������ڷ��Ӿ��壬ͬ�ַǽ���Ԫ��֮���γɷǼ��Լ���ͬ�ǽ���Ԫ��֮����ڼ��Թ��ۼ���
��3�������ͭ���ӷ�Ӧ���ɺ�ɫ����ͭ������
��4���Ȼ���Ͱ�����Ӧ�����Ȼ�泥�
����⣺��ǰ18��Ԫ����ɵ����ַ���A��B��C��D��E��������ԭ�Ӻ���Ŀ����Ϊ2��3��3��4��5������A��B������18�����ӣ�C��D��E������10�����ӣ���A��B��C��D��E�ֱ���HCl��H2S��H2O��NH3��CH4��
��1��D�ǰ��������������������ͣ�����������IJ��غϣ������Ǽ��Է��ӣ������ʽΪ��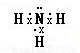 ���ʴ�Ϊ�����Է��ӣ�
���ʴ�Ϊ�����Է��ӣ�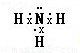 ��
��
��2��E�Ǽ��飬��������к����ĸ����۵������Ҳ����µ��Ӷԣ�����EΪ�������幹�ͣ��������ɷ��ӹ��ɵľ��壬���ڷ��Ӿ��壬C��ˮ��ˮ����ԭ�Ӻ���ԭ��֮����ڼ��Թ��ۼ���
�ʴ�Ϊ���������壬���ӣ����Թ��ۼ���
��3�������ͭ���ӷ�Ӧ������ͭ��ɫ��������Ӧ����ʽΪ��H2S+Cu 2+=CuS��+2H+��
�ʴ�Ϊ��H2S+Cu 2+=CuS��+2H+��
��4����A��D�ɰ�1��1�����������ӻ�����XΪ�Ȼ�泥��仯ѧʽΪ��NH4Cl��
�ʴ�Ϊ��NH4Cl��
���������⿼����ۺϣ��漰���ʽṹ�����ʣ���ȷ�ƶϻ������ǽⱾ��ؼ�����ȷ����10���ӡ�18�����ж�����Щ���ӣ�
��1��������ṹȷ�����Ӽ��ԣ�����ṹ�Գ������ڷǼ��Է��ӣ��������ڼ��Է��ӣ�
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ��ɷ��ӹ��ɵľ������ڷ��Ӿ��壬ͬ�ַǽ���Ԫ��֮���γɷǼ��Լ���ͬ�ǽ���Ԫ��֮����ڼ��Թ��ۼ���
��3�������ͭ���ӷ�Ӧ���ɺ�ɫ����ͭ������
��4���Ȼ���Ͱ�����Ӧ�����Ȼ�泥�
����⣺��ǰ18��Ԫ����ɵ����ַ���A��B��C��D��E��������ԭ�Ӻ���Ŀ����Ϊ2��3��3��4��5������A��B������18�����ӣ�C��D��E������10�����ӣ���A��B��C��D��E�ֱ���HCl��H2S��H2O��NH3��CH4��
��1��D�ǰ��������������������ͣ�����������IJ��غϣ������Ǽ��Է��ӣ������ʽΪ��
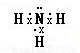 ���ʴ�Ϊ�����Է��ӣ�
���ʴ�Ϊ�����Է��ӣ�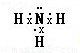 ��
����2��E�Ǽ��飬��������к����ĸ����۵������Ҳ����µ��Ӷԣ�����EΪ�������幹�ͣ��������ɷ��ӹ��ɵľ��壬���ڷ��Ӿ��壬C��ˮ��ˮ����ԭ�Ӻ���ԭ��֮����ڼ��Թ��ۼ���
�ʴ�Ϊ���������壬���ӣ����Թ��ۼ���
��3�������ͭ���ӷ�Ӧ������ͭ��ɫ��������Ӧ����ʽΪ��H2S+Cu 2+=CuS��+2H+��
�ʴ�Ϊ��H2S+Cu 2+=CuS��+2H+��
��4����A��D�ɰ�1��1�����������ӻ�����XΪ�Ȼ�泥��仯ѧʽΪ��NH4Cl��
�ʴ�Ϊ��NH4Cl��
���������⿼����ۺϣ��漰���ʽṹ�����ʣ���ȷ�ƶϻ������ǽⱾ��ؼ�����ȷ����10���ӡ�18�����ж�����Щ���ӣ�

��ϰ��ϵ�д�
�����Ŀ
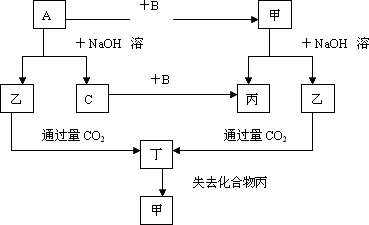
 ��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��
��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��