��Ŀ����
����Ŀ����.��1��������Һ�õ�������ע��Һ�������ǣ���ѧʽΪC6H12O6����ˮ��Һ�����ǩ�ϵIJ�����������ͼ��ʾ�����ñ�ǩ���ṩ����Ϣ�������ע��Һ�������ǵ����ʵ���Ũ��Ϊ____________ ��������λ��Ч���֣���

��2������˵�ѪҺ�������ǣ����Ѫ�ǣ��ĺ������ο�ָ�곣�����ּ�����λ��ʾ������mmol/L���͡�mg/dL��(1L=10dL)���ԡ�mmol/L����ʾʱ���˵�Ѫ������ֵ��3.61~6.11 mmol/L ֮�䡣��ij�˵�Ѫ�Ǽ����Ϊ92mg/dL������Ѫ��������_________ �������������������
��.����0.270kg��������Ϊ10%��CuCl2��Һ������
��1����Һ��CuCl2�����ʵ���Ϊ___________��
��2��Ҫ����Һ�е�Cl-��ȫ�����������1.0 mol/L��AgNO3��Һ_______mL��
��3��Ҫ����Һ�е�ͭ��ȫ�û������������Fe ������Ϊ__________��
���𰸡�0.28mol/L ���� 0.2mol 400 11.2g
��������
��.��1��C6H12O6��Ħ������Ϊ180g/mol��25g�����ǵ����ʵ���Ϊ25/180mol����Һ���Ϊ500 mL����ע��Һ�������ǵ����ʵ���Ũ��Ϊ(25/180)/0.5��0.28mol/L������������������ǣ�0.28mol/L��
��2��92mg/dL=(92��10-3)/180mol/dL=10��[(92��10-3)/180]mol/L=103��10��[(92��10-3)/180] mmol/L=5.11mmol/L, �˵�Ѫ������ֵ��3.61~6.11 mmol/L ֮��,���Ը���Ѫ������������������������ǣ�������
��.��1��0.270kg��������Ϊ10%��CuCl2��Һ��CuCl2�����ʵ���Ϊ��0.270��103��10%��/135=0.2mol ������������������ǣ�0.2mol��
��2�����ݣ�1����֪��0.2molCuCl2�к��������ӵ���Ϊ0.4mol�����ݷ�ӦAg++Cl-=AgCl����Ӧ��֪�����������ӵ���Ϊ0.4mol�����ݹ�ʽ��n=c��V��֪��1.0��V=0.4��V=0.4L=400 mL������������������ǣ�400 ��
��3�����ݷ�Ӧ��Fe+Cu2+=Fe2++Cu��֪��0.2molCuCl2�к���Cu2+0.2mol������������Ϊ0.2mol������Ϊ0.2��56=11.2g������������������ǣ�11.2g��

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�����Ŀ������ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ����ȷ����
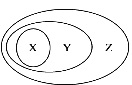
X | Y | Z | |
A | ˮ | ���� | ������ |
B | �ǽ��������� | ���������� | ������ |
C | ����� | ������ | ������ |
D | ���Ϸ�Ӧ | ������ԭ��Ӧ | ��ѧ��Ӧ |
A. A B. B C. C D. D