��Ŀ����
8��ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϣ���1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊֱ��Һ�������ͼ��Һ��������
��2��ú������һ�����˵ġ��Ƽ������ϸߵ�ú������������չú���Ͷ��ҹ����Ծ����ش����壮������ú�����ͺͱ�ϩ����Ҫ��������ͼ��
��Ϊ�����ԭ�������ʣ�����������Ӧ���ƺϳ�����V��CO����V��H2��=1��2��
�ڲ���MTG�������������У����ļױ���1��2��4��5-�ļ�������������Լռ4%��7%�����ļױ��Ľṹ��ʽΪ��
 ��
����3������ú�����������ɻ�úϳɰ���ԭ������������ȡԭ�����Ĺ����г�����һЩ���ʣ�����ijЩ���ʻ�ʹ�����ж��������ȥ��
��д����ȥH2S���ʵķ�Ӧ����ʽNH3•H2O+H2S=NH4HS+H2O��
������ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO��g��+H2O��g��?CO2��g��+H2��g������֪850��ʱ�÷�Ӧ��ƽ�ⳣ��K=1����ҪʹCO��ת���ʳ���90%������ʼ����c��H2O����c��CO��������9��1��
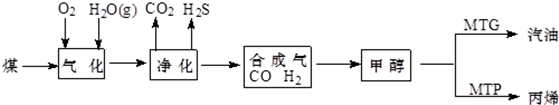
��4��ú����Դ��ȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
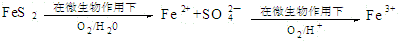
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ2FeS2+7O2+2H2O=4H++2Fe2++4SO42-���ڶ�����Ӧ�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O��
���� ��1��ú��Һ����Ϊֱ��Һ���ͼ��Һ����
��2����д����Ӧ�ķ���ʽ�����ݷ���ʽ���㣻
�ڸ������ƿ�д���ṹ��ʽ��
��3�����е����ʻ�ʹ�����ж���
�ٸ����������������壬��H2S���������������������Լ���
�ڸ���ƽ�ⳣ��������ʽ�����㣮
��4�����ݻ��ϼ�������������Լ�ԭ���غ���д���ӷ���ʽ��
��� �⣺��1��ú��Һ����Ϊֱ��Һ���ͼ��Һ����ú��ֱ��Һ���ǽ�ú�������ʹ���������ͨ�������ѻ�ת��ΪҺ��ȼ�ϵĹ��̣��������Ҫ���ü����ֶΣ����ֳ�ú�ļ���Һ������ú�ļ��Һ�����Ȱ�ú̿�ڸ�������������ˮ������Ӧ��ʹú̿ȫ��������ת���ɺϳ�����һ����̼�������Ļ�����Ȼ�����ڴ����������ºϳ�ΪҺ��ȼ�ϵĹ��ռ������ʴ�Ϊ��ֱ��Һ�����������Һ��������
��2���ٺϳɼ״��ķ���ʽΪCO+2H2��CH3OH���ɷ���ʽ��֪����������Ӧ���ƺϳ�����V��CO����V��H2��=1��2��
�ʴ�Ϊ��1��2��
�ڸ������ƿ�֪���ļױ��Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3�����е����ʻ�ʹ����ʧЧ���������ж����ʴ�Ϊ���ж���
�ٸ����������������壬��H2S�����������֪�����ð�ˮ���������Լ�����ӦΪ��NH3•H2O+H2S=NH4HS+H2O���ʴ�Ϊ��NH3•H2O+H2S=NH4HS+H2O��
����CO����ʼŨ��Ϊxmol��H2O����ʼŨ��Ϊymol����ת����CO����СֵΪ0.9x��
CO��g��+H2O��g��?CO2��g��+H2��g��
��ʼ x y 0 0
�仯 0.9x 0.9x 0.9x 0.9x
ƽ�� 0.1x y-0.9x 0.9x 0.9x
����ƽ�ⳣ����ʽ��$\frac{��0.9x��^{2}}{0.1x��y-0.9x��}$=1��
��ã�$\frac{y}{x}$=9��1���ʴ�ֻҪ��ֵ����9��1��ת���ʾͻᳬ��90%��
�ʴ�Ϊ��9��1��
��4����һ����Ӧ�з�Ӧ����FeS2��O2��H2O����������Fe2+��SO42-�����ݻ��ϼ�������������Լ�ԭ���غ㣬��Ӧ�����ӷ���ʽΪ��2FeS2+7O2+2H2O=4H++2Fe2++4SO42-��Fe2+���л�ԭ�ԣ��ɱ���������ΪFe3+�����ݻ��ϼ�������������Լ�ԭ���غ㣬��Ӧ�����ӷ���ʽΪ��4Fe2++O2+4H+=4Fe3++2H2O��
�ʴ�Ϊ��2FeS2+7O2+2H2O=4H++2Fe2++4SO42-��4Fe2++O2+4H+=4Fe3++2H2O��
���� ���⿼��ú���ۺ����ã��漰��������ԭ����ʽ����д����Ŀ�Ѷ��еȣ�����ע�����Ŀ�л�ȡ��Ϣ��������ʵ����ʽ��

 ������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ����NO2����������������ǣ�������
������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ����NO2����������������ǣ�������| A�� | aΪֱ����Դ������ | |
| B�� | �����ĵ缫��ӦʽΪ��2HSO3-+e-=S2O42-+H2O | |
| C�� | �����ĵ缫��ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+ | |
| D�� | ���ʱ��H+��������ͨ�������ӽ���Ĥ���������� |
| A�� | ��KSCN��Һ����Fe2+ | B�� | ��ʪ��ĺ�ɫʯ����ֽ���鰱�� | ||
| C�� | ��ʪ��ĵ��۵⻯����ֽ�������� | D�� | �������ữ��AgNO3��Һ����Cl- |
 ±����AgX��Ag2Z�ij����ܽ�ƽ��������ͼ��ʾ����֪������p��Ag+��=-lgc��Ag+����������y=-lgc��X-����-lgc��Z2-��������˵����ȷ���ǣ�������
±����AgX��Ag2Z�ij����ܽ�ƽ��������ͼ��ʾ����֪������p��Ag+��=-lgc��Ag+����������y=-lgc��X-����-lgc��Z2-��������˵����ȷ���ǣ�������| A�� | ���¶���Ag2Z��KspԼΪ1��10-8 | |
| B�� | a��ɱ�ʾAgCl�Ĺ�������Һ | |
| C�� | b��ɱ�ʾAgI�Ĺ�������Һ����c��Ag+��=c��I-�� | |
| D�� | ���¶���AgCl��AgBr�γɵĻ����Һ�ı�����Һ�У�c��Cl-����c��Br-�� |
| A�� | ��FeI2��Һ��ͨ������ʵ�����Cl2��2I-+Cl2�T2Cl-+I2 | |
| B�� | �ù�����ˮ�����̵����е�SO2��SO2+NH3•H2O�THSO3-+NH4+ | |
| C�� | ��NaHSO4��Һ�м���Ba��OH��2�����ԣ�H++SO42-+Ba2++OH-�TH2O+BaSO4�� | |
| D�� | ��AgCl������Һ�еμ�Na2S��Һ��2AgCl+S2-�TAg2S+2Cl- |
| A�� | ��������60 | B�� | �������� 27 | C�� | ��������33 | D�� | ��������33 |
 ������۲���ͼ��Ȼ��ش����⣮
������۲���ͼ��Ȼ��ش����⣮

