��Ŀ����
8��W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ�X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ�Y2+��X2-������ͬ�ĵ��ӹ��ͣ�Z��ԭ������Ϊ28����1��Zԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
��2����ͬ���ڵ�����Ԫ�رȽϣ�Yԭ�ӵĵ�һ�����ܽϴ���ϴ�С������ԭ����þ�ļ۵����Ų���3s2���ﵽS�Dz��ȫ����״̬��������ԭ�ӱȽϣ�Mgԭ������ȶ���
��3��WX2�����У����ۼ��������ЦҼ��ͦм���Wԭ�ӵ��ӻ��������Ϊsp�ӻ���WX32-����ԭ���ϵŶԵ��Ӷ���Ϊ0�����幹��Ϊƽ�������Σ�д��WX32-������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ����ӣ�SO3��NO3-��д���֣�
��4��������YX�ľ���ṹ��NaCl��ͬ��Y����λ����6�����۵���ڣ�����ڡ��������ڡ���Լ���ڡ���NaCl��ԭ������NaCl������ȣ�MgO���������ӵĵ�����뾶С�����MgO�ľ����ܴ���NaCl�ľ����ܣ��Ӿ����۽ṹ�ĽǶȽ��ͣ���
��5����W��Y��Z����Ԫ����ɵ�һ�ּ������ṹ�Ļ�������г����ԣ��侧����Wλ������λ�ã�Yλ�ڶ��ǣ�Zռ������λ�ã��û�����Ļ�ѧʽΪMgCNi3��������Yԭ����Χ���������Zԭ����12�������ͳ������Ͼ�������a=0.3812nm����ʽ����þ�����ܶȣ�g•cm-3��6.029g•cm-3��
���� W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������
Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ���W�ĵ����Ų�ʽΪ1s22s22p2����WΪCԪ�أ�
X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ���X�����Ų�ʽΪ1s22s22p4����XΪOԪ�أ�
Y2+��X2-������ͬ�ĵ��ӹ��ͣ���YΪMgԪ�أ�
Z��ԭ������Ϊ28����ZΪNiԪ�أ��ݴ˽��
��� �⣺W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������
Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ���W�ĵ����Ų�ʽΪ1s22s22p2����WΪCԪ�أ�
X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ���X�����Ų�ʽΪ1s22s22p4����XΪOԪ�أ�
Y2+��X2-������ͬ�ĵ��ӹ��ͣ���YΪMgԪ�أ�
Z��ԭ������Ϊ28����ZΪNiԪ�أ�
��1��ZΪNiԪ�أ�ԭ�ӵļ۲�����Ų�ʽΪ3d84s2���ʴ�Ϊ��3d84s2��
��2��þ�ļ۵����Ų���3s2���ﵽS�Dz��ȫ����״̬��������ԭ�ӱȽϣ�Mgԭ������ȶ�������ʧ���ӣ���һ�����ܽϴʴ�Ϊ���ϴ�þ�ļ۵����Ų���3s2���ﵽS�Dz��ȫ����״̬��������ԭ�ӱȽϣ�Mgԭ������ȶ���
��3��CO2���ӵĽṹʽΪO=C=O�������ۼ�������Ϊ�Ҽ��ͦм���CO2��������ԭ��C���Ӷ���Ϊ$\frac{1}{2}$��4+0��=2�����ӻ���ʽΪsp�ӻ���CO32-��������ԭ����̼ԭ�ӣ�̼ԭ�������4������ȫ�����ڳɼ���û�йµ��Ӷԣ��۲���Ӷ���=�Ҽ����Ӷ���+�µ��Ӷ�����CO32-������ԭ�ӵļ۲���Ӷ���Ϊ3��VSEPRģ������Ϊƽ�������Σ�
��CO32-������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ�����Ϊ�ȵ����壬Ӧ����4��ԭ�ӣ��۵�����Ϊ24��������SO3��NO3-��
�ʴ�Ϊ���Ҽ��ͦм���sp�ӻ���0��ƽ�������Σ�SO3��NO3-��
��4��������MgO�ľ���ṹ��NaCl��ͬ���������������ͣ�Mg����λ����6����NaCl������ȣ�MgO���������ӵĵ�����뾶С�����MgO�ľ����ܴ���NaCl�ľ����ܣ��ʴ�Ϊ��6����NaCl������ȣ�MgO���������ӵĵ�����뾶С�����MgO�ľ����ܴ���NaCl�ľ����ܣ�
��5��������̼λ�����ģ�ֻ��һ����þλ�ڽ��ϣ�ÿ��þԭ�ӱ�8���������ã���ÿ��������þԭ�Ӹ���Ϊ8��0.125=1������λ�����ģ�ÿ����ԭ�ӱ������������ã���ÿ����������ԭ�Ӹ���Ϊ6��0.5=3�����ʾ��廯ѧʽΪMgCNi3��ÿ��������þ��Χ��3����ԭ�ӣ�ÿ��þ���˸��������ã��Ϸ����ĸ���������������ԭ�ӣ��ԳƵ��·�Ҳ��������һ��12����������ܶȦ�=$\frac{m}{V}$=$\frac{\frac{M}{{N}_{A}}}{{a}^{3}}$=$\frac{��12+59��3+12��g/mol}{��0.3812��1{0}^{-7}cm��^{3}��6.02��1{0}^{23}mo{l}^{-1}}$=6.029g•cm-3��
�ʴ�Ϊ��MgCNi3��12��6.029g•cm-3��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�á������ļ��㣬��Ŀ�Ѷ��еȣ������ƶϸ�Ԫ��Ϊ���ؼ���ע������Ԫ�����ڱ��ṹ��Ԫ�������ɵ����ݣ���ȷ������������ѻ���ʽ������֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ�����ѧ�����Ӧ�û���֪ʶ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �٢ڢۢ� | B�� | �ڢۢܢ� | C�� | �ܢۢڢ� | D�� | �ڢۢ٢� |
| A�� | ���ȱ������Ȼ�����Һ�Ʊ������������壺Fe3++3H2O$\stackrel{��}{?}$Fe��OH��3�����壩+3H+ | |
| B�� | ��������ӵĵ��뷽��ʽ��HS-+H2O?H3O++S2- | |
| C�� | ��������������Һ��̼��������Һ��ϵ����ӷ���ʽ��Ca2++2HCO3-+2OH-�TCaCO3��+2H2O+CO32- | |
| D�� | 0.5mol•L-1��K2Cr2O7��Һ�д�������ƽ�⣺Cr2O72-+H2O?2CrO42-+2H+����ƽ�ⳣ��K�ı���ʽΪK=$\frac{{c}^{2}��Cr{{O}_{4}}^{2-}��•{c}^{2}��{H}^{+}��}{c��C{r}_{2}{{O}_{7}}^{2-}��}$ |
| A�� | 64gCu��������ʧȥ�ĵ�����һ��Ϊ2NA | |
| B�� | �����£�pH=13�İ�ˮ�У���ˮ�����OH-��Ϊ0.1NA | |
| C�� | �ڱ�״���£�22.4LC4H10�й��ۼ���ĿΪ13NA | |
| D�� | 200mL1mol/LFe2 �� SO4��3 ��Һ�У�SO42-��������0.3NA |
| A�� | ��Ӧ���Ȼ�ѧ����ʽΪ2NH4Cl+Ba��OH��2�TBaCl2+2NH3•H2O����H��0 | |
| B�� | ��Ӧ�������������������������� | |
| C�� | �Ȼ�������������ķ�ӦΪ���ȷ�Ӧ | |
| D�� | �÷�Ӧ�У���ѧ��ȫ��ת��Ϊ���� |
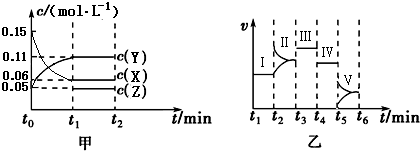
 2Y��g��+Z��g����H=-10akJ/mol������ͼ����ƽ���У�ƽ�ⳣ�������Ǣ���
2Y��g��+Z��g����H=-10akJ/mol������ͼ����ƽ���У�ƽ�ⳣ�������Ǣ���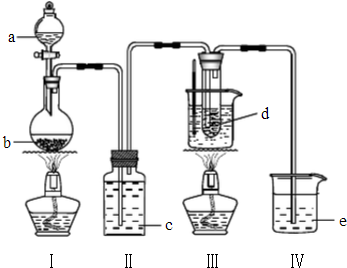 ���������Һ��Ӧʱ���ڵ��º�ϡ����Һ����Ҫ������ClO-��Cl-����75�����Ϻ�Ũ����Һ����Ҫ������ClO-��Cl-���о�С��������ʵ��װ����ȡ����أ�KClO3�����ⶨ�ܶȣ�
���������Һ��Ӧʱ���ڵ��º�ϡ����Һ����Ҫ������ClO-��Cl-����75�����Ϻ�Ũ����Һ����Ҫ������ClO-��Cl-���о�С��������ʵ��װ����ȡ����أ�KClO3�����ⶨ�ܶȣ� ��1����ҵ������ˮ�������������Ƶ��������£�
��1����ҵ������ˮ�������������Ƶ��������£�